Drug Pricing
Our work in Drug Pricing
-
The Urgent Need for a New Generation of Antibiotics
Health policy experts discuss how to ensure the development of new antimicrobials to replace antibiotics and other critical medications that are beginning to lose their effectiveness.
-
Shame Won’t Solve America’s Obesity Crisis: How Congress Can Help
If saving lives is the objective, then logic, clinical evidence and compassion dictate that Medicare should pay for preventing and treating obesity, starting now.
Categorized in -
The Inflation Reduction Act Is Already Killing Potential Cures
The law’s price controls do away with incentives for research and development of life-saving drugs.
Categorized in -
CMS’s Alzheimer’s Coverage Policy Will Inhibit Access and Discourage Innovation
Individuals in underserved communities have to travel long distances, well beyond the 30-minute standard, to access clinical trial sites for Aduhelm.
Categorized in -
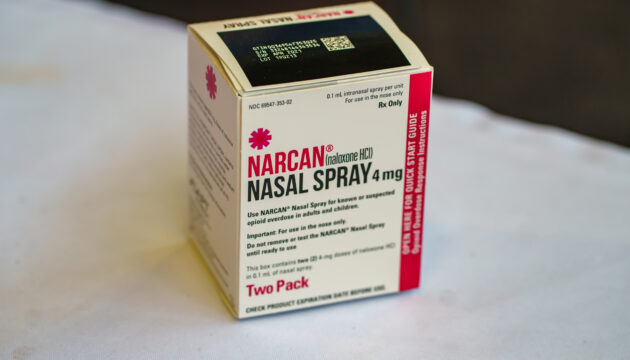
During the Height of the Opioid Epidemic, the Out-of-Pocket Price of Naloxone Increased by Over 500%
A jump in the out-of-pocket price of naloxone has likely made the lifesaving drug too expensive for most uninsured Americans.
Categorized in -
Drugmakers Aren’t Driving Inflation. Price Controls Would Hinder Medical Progress
Drug prices have not experienced the major rise in cost like eggs or gasoline, and instituting price controls on drugs would only stifle medical innovation.
Categorized in -
Reforming the Medicare Part D Benefit Design: Financial Implications for Beneficiaries, Private Plans, Drug Manufacturers, and the Federal Government
Researchers evaluate prescription drug claims from Medicare Part D beneficiaries to evaluate the current spending distribution.
Categorized in -
The Cantwell-Grassley PBM Bill Is Much Needed But More Can Be Done
The proposed reforms are a helpful start, but could go further in addressing the lack of competition in the U.S. PBM market.
Categorized in -
PBMs Are Inflating the Cost of Generic Drugs. They Must Be Reined In
Anti-competitive practices and a lack of transparency in the generic drug market allow pharmacy benefit managers to profit off patients and payers.
Categorized in -
The FDA Could Do More to Promote Generic Competition: Here’s How
USC-Brookings Schaeffer Initiative experts focus on three areas of FDA authority that could be refined to better promote generic competition: the Citizen Petition mechanism; the approval of so-called complex generic drugs; and the phenomenon known as “parking” under the Hatch-Waxman Act.
Categorized in