Research Program
Healthcare Markets Initiative
U.S. healthcare markets suffer from both overuse and underuse. The challenge is developing the appropriate incentives that eliminate the inefficiencies which lead to some services being too expensive and reimbursements for other services being too low. The Healthcare Markets Initiative advances market-based solutions to health policy challenges in a variety of areas including rare diseases, medical devices and mental health.
Program Leadership
-

Matthew Kahn, PhD
Director, Healthcare Markets Initiative, USC Schaeffer Center
Senior Scholar, USC Schaeffer Institute
Provost Professor of Economics and Spatial Sciences, USC Dornsife College of Letters, Arts, and Science
Featured Research

Mitigating the Inflation Reduction Act’s Adverse Impacts on the Prescription Drug Market
This paper provides three recommendations to steer the potential effects of the IRA toward its goal of improving patient access while encouraging innovation.

Biosimilar Drugs Underutilized Due to Commercial Insurance Restrictions
The first study to examine biosimilar drivers finds commercial insurers limit use in almost 20% of cases.

Macroeconomic Consequences of the COVID-19 Pandemic
We estimate the economic impacts of COVID-19 in the U.S. using a disaster economic consequence analysis framework implemented by a dynamic computable general equilibrium (CGE) model.
Benefits of Medicare Coverage for Weight Loss Drugs
The cumulative social benefits from Medicare coverage for new obesity treatments over the next 10 years would reach almost $1 trillion, or roughly $100 billion per year.
Healthcare Markets Researchers
-

Joseph Grogan, JD
Nonresident Senior Scholar, USC Schaeffer Institute
Former Assistant, U.S. President
Former Director, Domestic Policy Council (DPC)
-

Darius Lakdawalla, PhD
Chief Scientific Officer, USC Schaeffer Center
Senior Scholar, USC Schaeffer Institute
Quintiles Chair in Pharmaceutical Development and Regulatory Innovation, USC Mann School
Professor, USC Price School of Public Policy
Medical Device Regulation and Reimbursement: What the Pandemic Revealed
Schaeffer Center Nonresident Senior Fellow and Former Director of the Domestic Policy Council Joe Grogan and AEI Senior Fellow and Former FDA Commissioner Scott Gottlieb joined Schaeffer Center Co-Director Erin Trish to discuss the challenges and opportunities facing the FDA and CMS in an age of breakthrough medical devices.
Read Full StoryFeatured Perspectives
The Agency Keeping Alzheimer’s Drugs from Patients
The Centers for Medicare and Medicaid Services refuses to approve breakthrough drugs.
Four Ways to Make Drug Price Negotiations Work for Everyone
The IRA will be measured not just by how much it can lower prices, but also by whether it continues to encourage drug makers to invest in new treatments.
Medicare’s ‘Coverage with Evidence Development’: A Barrier to Patient Access and Innovation
CMS should abandon CED or, at minimum, reform and restrict its use only for off-label applications of therapies.

A Strategy for Value-Based Drug Pricing Under the Inflation Reduction Act
To make sure that CMS is getting its money’s worth for today’s drugs, it must ensure that the maximum fair price is aligned to drug value.
Medicare Advantage Penetration Has Increased Across Rural and Urban Counties
All told, in 2022 MA penetration reached 49.9% nationally, and 24% of Medicare beneficiaries with Parts A and B lived in a county with adjusted MA penetration equal to or exceeding 60%.
Read Full Story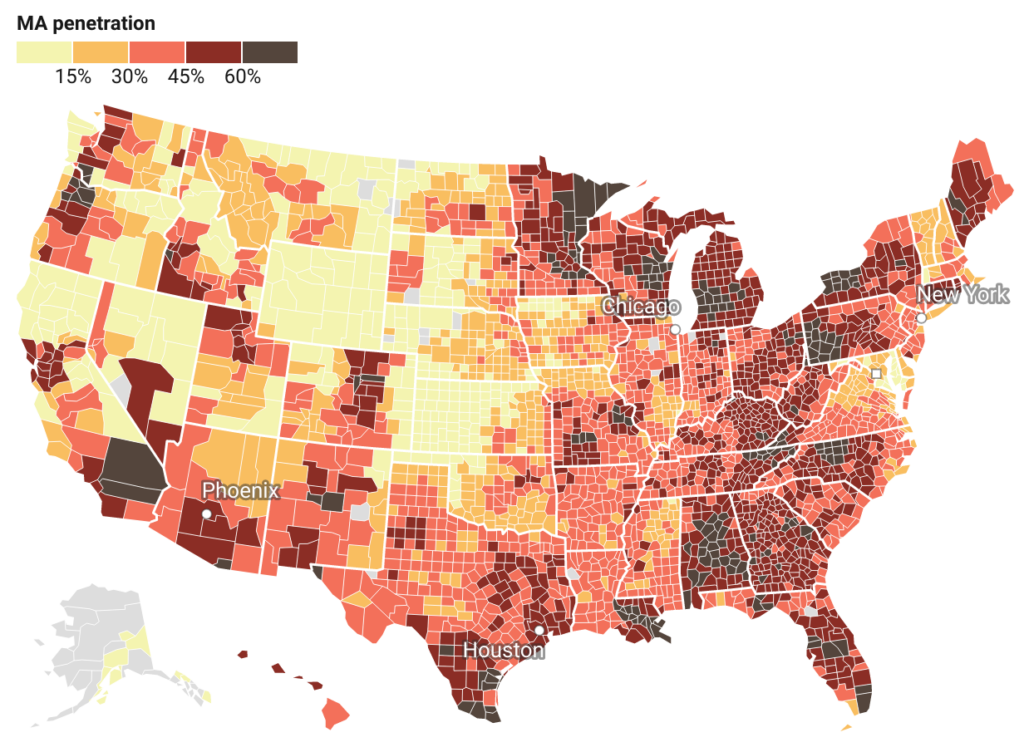
Get Involved
Learn more about HMI, including opportunities to collaborate