The USC Schaeffer Center for Health Policy & Economics and the Aspen Institute have together established an advisory panel to consider how the U.S. can better link the price of health technologies to the benefits they provide to patients while ensuring a sustainable healthcare ecosystem that supports innovation. Experts on value assessment are convening for meetings in October 2019 and April 2020 to develop policy recommendations that support this ecosystem. While the U.S. can learn from other countries’ implementation of Health technology assessment, the panel’s aim is to make practical recommendations that are tailored to the unique U.S. healthcare system and can garner broad support.
Background
In 2016, medical care spending in the United States exceeded that of other high-income countries by a factor of two, yet the U.S. population has not attained commensurate health outcomes.[1] Studies suggest that higher prices, rather than greater utilization or better quality of care, drive higher U.S. spending.[2-4] While drug prices are often blamed for higher healthcare costs,[5] drugs account for 15 percent of U.S. healthcare spending,[6] while hospital and physician services account for 52 percent.[7] Therefore, services cannot be omitted from discussions about how to reduce spending. A natural question arising from such discussions is whether the prices of health technologies, including drugs and medical devices, as well as services and procedures, reflect their benefit to patients.
Health technology assessment (HTA) provides a framework to determine whether prices of health interventions reflect their benefits to patients. HTAs can be implemented in various ways depending on the goals and preferences of a health system or payer, but consist of two key components—assessment and appraisal—which are often conducted by different entities. The assessment portion of HTA involves systematic review and evaluation of scientific evidence, such as clinical outcomes or economic costs associated with a technology. The appraisal portion of HTA uses the evidence assembled during assessment to develop coverage recommendations. While these recommendations are usually used in pricing decisions or negotiations between payers and manufacturers (which occur subsequent to the HTA process), other factors—such as input from patient groups—might also influence final price or coverage decisions.
In contrast to many developed countries, the U.S. does not have a national HTA program to broadly evaluate health technologies and guide coverage and pricing decisions.[i] The lack of a single national HTA organization or process reflects the current U.S. political landscape—including our preference for market-oriented solutions—as well as our decentralized insurance system, under which each private and public payer makes its own coverage decisions and conducts its own price negotiations. While U.S. payers frequently use internal processes that incorporate elements of HTA to inform their coverage decisions, these processes lack transparency and involve duplicated efforts across organizations. At the same time, shifting to a single national approach to HTA would be challenging in the U.S. given differences in covered populations across payers.
This paper summarizes a longer background report that was prepared for panelists before the first convening of the advisory panel, available here. It reviews select existing HTA efforts and provides context for developing a more systematic HTA framework for the U.S.
Historical and Political Context
Although the U.S. currently lacks a national HTA program to guide coverage and pricing decisions, this has not always been the case (Figure 1; see background report for further details).[ii] The first example of an official U.S. HTA organization was the Office of Technology Assessment (OTA).[iii] Established in 1972, the OTA was created to inform Congress of the impact of new technologies and included a program focused specifically on healthcare. Critiques of this program included concerns about healthcare rationing and reduced access to new technologies, as well as fears that it would threaten innovation and physician autonomy.[8] Several issues that dogged OTA during its existence continue to challenge U.S. governmental agencies and government-financed organizations today. The OTA suffered criticism of partisanship after it negatively reviewed Reagan’s Strategic Defense Initiative (aka “Star Wars” missile defense). Subsequently, Congress became concerned about OTA’s potential to attenuate its decision-making power and increasingly wanted the agency to reflect its interests.[13] The OTA was eventually dismantled in 1995 when the Republican-led legislature identified it as bureaucratic waste and eliminated its funding, which amounted to one percent of the Congressional budget.
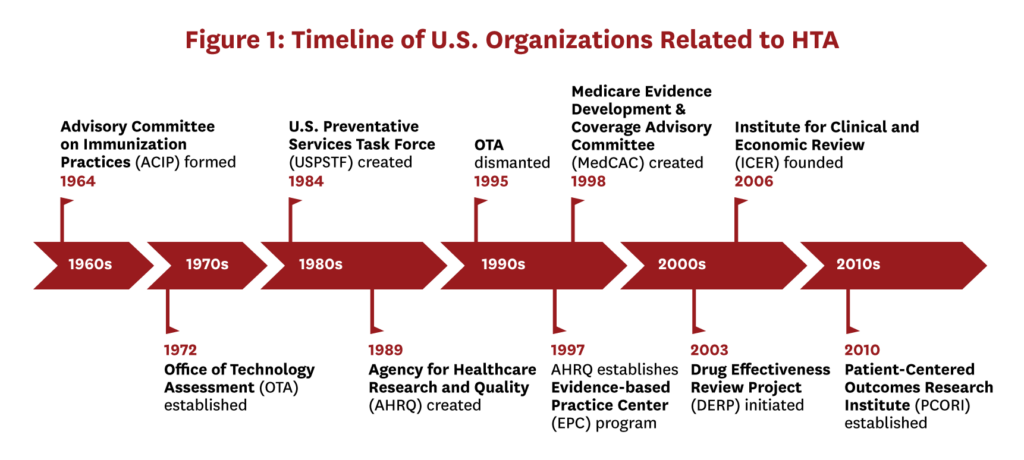
Several agencies in the U.S. still undertake more limited health technology evaluation. The Agency for Healthcare Research and Quality (AHRQ) conducts HTA through various programs, including its Evidence-based Practice Center (EPC) program, and the Department of Veterans’ Affairs conducts assessments to guide coverage. At the state level, Medicaid agencies initiated the Drug Effectiveness Review Project (DERP) in 2003 with the goal of creating comparative effectiveness reviews, and several states use DERP reports as primary evidence to determine coverage. More recently, the Patient-Centered Outcomes Research Institute (PCORI) was established in 2010,[14] with the goal of focusing health research on patient concerns. While PCORI does not conduct HTAs or systematically evaluate new technologies, it funds comparative effectiveness research and aims to include patients throughout research processes. PCORI has received bipartisan support, and in 2019 Congress renewed its funding for ten more years.[15]
In the absence of a governmental HTA body in the U.S., the Institute for Clinical and Economic Review (ICER) has emerged to evaluate new therapies. Founded in 2006, ICER is an independent non-profit organization that evaluates the clinical and economic value of prescription drugs, medical tests, and other healthcare innovations. ICER’s profile was elevated in 2015 when they initiated the Emerging Therapy Assessment and Pricing program, funded through a $5.2 million grant from the Laura and John Arnold Foundation.[16] ICER consists of three appraisal committees, each of which conducts two to five evaluations annually.[iv]
Methodological Considerations for Health Technology Assessment[v]
The methodological scope of HTAs varies widely; it may consider clinical impact alone or incorporate economic analyses and other factors. Absent cost constraints, an HTA that focuses solely on clinical outcomes such as efficacy and safety will provide the information necessary to choose among clinically relevant alternatives. However, because resources are limited, the economic value of health technologies has become an increasingly important factor in decision-making. Payers must balance providing access to effective health interventions with their costs. HTAs that incorporate economic evidence aid in identifying those interventions whose prices are justified by the benefits they deliver and which may be overpriced.[vi]
To inform potential approaches to HTA in the U.S., we reviewed the HTA processes used in Australia, Canada, France, Germany, Japan, and the United Kingdom (U.K.). Figure 2 presents a continuum of approaches to implementing an HTA and examples where each is used. Each of the countries reviewed conducts HTAs following regulatory approval of a drug if the manufacturer plans to obtain marketing approval for inclusion on the (usually government-run) insurance plan (see background report for further country-specific details). The approaches range from no HTA process (i.e., a drug does not undergo additional evaluation after regulatory approval) to one that evaluates all key elements (clinical, economic, and budget impact). In addition, HTAs might include other considerations that are not unique to any one approach. Specifically, contextual factors may be incorporated, and exceptions might be made for certain drugs (e.g., cancer, rare diseases) or populations (e.g., pediatric, end-of-life). Finally, HTA processes may vary in their approach to transparency and stakeholder engagement.
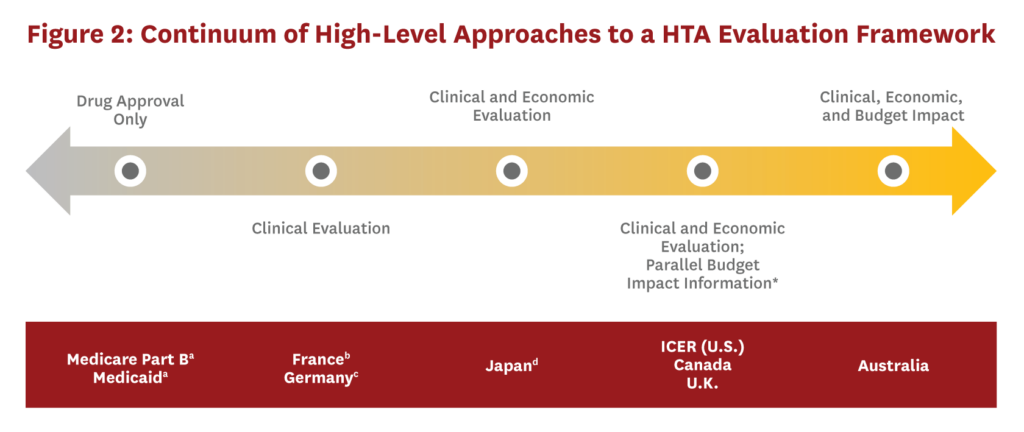
If the U.S. were to create an official HTA organization, it would first need to determine which elements to include in assessments. Inclusion of economic information is likely to face strong opposition in the U.S., therefore an approach that only considers clinical benefit may be a more pragmatic first step. However, an HTA process that excludes an economic evaluation fails to provide guidance on whether drug prices reflect the benefits provided. Additional methodological decisions would also be required, such as choosing which health interventions to evaluate and the timing of the HTA process. Finally, if the U.S. opted to create a national HTA body, its efforts could make some of the individual HTA evidence-generating efforts currently underway unnecessary. The current role of the private sector in generating HTA evidence would need to be reconsidered and possibly recast in the face of a national HTA effort.
The success of any official HTA body in the U.S. will also require that issues of transparency and stakeholder engagement are adequately addressed. While a range of stakeholders should have some degree of engagement during the HTA process, examples from other countries show no consensus on which stakeholders should have a voting role in appraisals. Manufacturers are excluded from the voting body in most countries, but submit clinical and economic evidence to the HTA process and are represented during price negotiations (separate from the HTA). While patient involvement in the HTA process has increased, very few countries give patients voting rights. The ultimate degree of stakeholder involvement in the U.S. should reflect the goals and principles of the HTA organization.
The HTA process provides information regarding the level of clinical or economic benefit of a technology, but in most countries, final pricing decisions are made in a separate step after the HTA process. Moreover, HTA results may be just one of several inputs to the pricing decision; other regulatory mechanisms independent of HTA, such as reference pricing, may factor into the final price. The next section summarizes some ways that HTA information might be used for pricing decisions in the U.S. While we focus on drug pricing, the discussion could apply more broadly to other health technologies or services.
Using Health Technology Assessment to Inform Drug Pricing[vii]
Economists agree that drug prices should reflect the value of treatments to patients and society. If drug prices are set too low relative to their benefits, manufacturers may be reluctant to invest in research and development, thereby stifling future innovation. But prices set too high stimulate inefficient levels of innovation, and affordability issues may limit patient access. Yet prices for drugs in the U.S. often seem decoupled from value, which is largely the result of complex commercial dynamics in the current U.S. drug marketplace.
Private and government plans set their own drug provision and pricing policies in the U.S. Formulary and pricing decisions are decentralized as each plan negotiates separately and out of the public eye. Moreover, multiple entities in the supply chain including insurers, manufacturers, wholesalers, pharmacies, and pharmaceutical benefit managers (PBMs) influence drug prices. Insured consumers rarely face the full price of a drug, but are responsible for a co-pay or coinsurance; additionally, the presence of intermediaries means that manufacturers do not necessarily face declines in consumer demand when prices rise. Prices ultimately depend on the relative bargaining position of buyers (insurers/PBMs) and sellers (manufacturers) and tend to be linked to volume.
One or more of these features might explain why U.S. drug prices do not consistently reflect their benefits and why there are growing calls for value-based pricing. While insurers in the U.S. may currently use value assessment or economic evaluations in coverage decisions or drug price negotiations, such practices are not transparent to consumers.19-22 Further, rising drug prices in the U.S. have prompted calls for government intervention to regulate them, begging the question: Would government-set drug prices be more closely tied to value?[viii,23]
Even if the U.S. decides drug pricing should incorporate HTA information and a national HTA body could play a role, it will need to determined how to ensure prices reflect value. Important elements of such a process include defining and incorporating value into pricing, and determining methods of price setting, either negotiating or setting them based on a formula. If prices are determined through negotiations, it will need to be decided which parties participate, whether negotiations are bilateral or multilateral, and what happens when negotiations fail.
Alternative approaches to linking HTA results to pricing decisions could be considered (Figure 3). They range from market-oriented options that allow discretionary price negotiation by payers to more formulaic options that limit pricing discretion by public or private payers. Although an advisory-only system is the most market-oriented approach on the continuum, it contains few, if any, mechanisms to ensure prices reflect value. Moreover, an advisory-only HTA body will not eliminate market inefficiencies resulting from the current pharmaceutical supply chain.
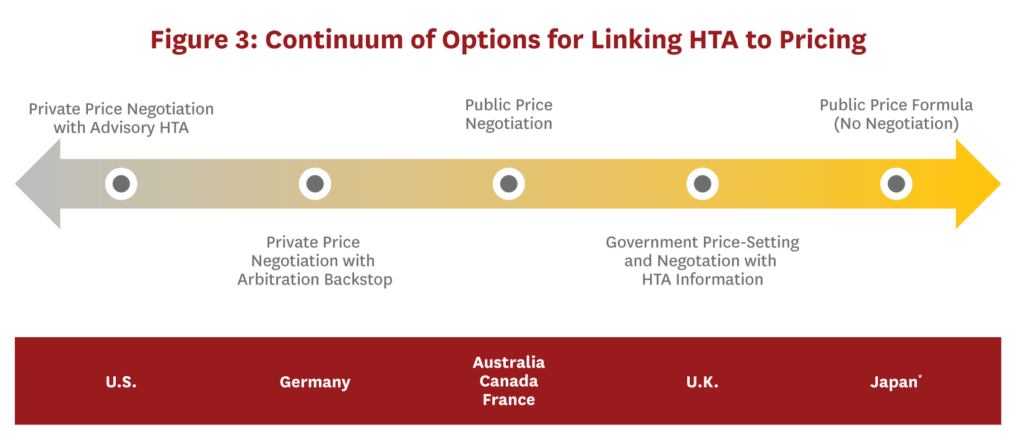
Moving toward the right along the continuum reveals more centralized price negotiations based on HTA recommendations. In implementing these options, the U.S. would need to consider whether private insurers or the government, with its greater leverage, would negotiate prices. Even if the government assumes responsibility for negotiations, drug coverage could still be administered through private insurers. Additionally, if prices are negotiated, there must be some price-setting default in case negotiations fail, and mechanisms to ensure the resulting prices are not too low to sustain valuable innovation. An arbitration backstop would prevent manufacturers from exiting the market entirely or limiting access to only those patients who pay with cash or have supplemental insurance if negotiations fail.[ix] Alternatively, statutory regulations could further backstop against drug prices that are “too high.”[x]
A final possibility along the continuum would eliminate price negotiations entirely and tie prices to HTA results using a formula. Although price setting by formula is more restrictive than negotiations, it provides a clearly defined and transparent process for linking HTA recommendations (and presumably value) to drug prices. However, such an approach assumes a single correct price exists and all relevant elements for deriving that price are known and accounted for in the formula. Alternatively, rather than using a formula, coverage could be determined using a cost-effectiveness threshold. Even using thresholds, flexibility in coverage determinations and prices could be introduced through variable thresholds and/or exceptions for certain disease areas or populations.
What is Cost-Effectiveness Analysis?
Cost-effectiveness analysis (CEA) is a key input in many HTAs that enables the incorporation of economic information (or value) of health gains into the assessment. Cost-effectiveness is calculated by dividing the difference in total costs between two interventions by the difference in health. A widely accepted measure of health is the quality-adjusted life year (QALY), which accounts for both the quality and quantity of life associated with a health intervention.
Decision makers may select a threshold for cost effectiveness when evaluating new health interventions, which is the maximum amount of money they would be willing to spend for an additional QALY. Interventions with costs below the threshold are considered cost-effective and are more likely to be recommended for coverage in HTA processes that incorporate economic evidence.
Irrespective of the particular approach used to link HTA recommendations to pricing, decision makers will need to address many trade-offs and challenges. A balance must be struck between priorities and budget constraints of the health system or payers and preferences of other stakeholders, who may have different objectives with respect to drug access or price setting. Other potential trade-offs include balancing HTA timing against delayed drug access, transparency versus flexibility in price setting, and weighing the benefits of efficiency gains from a more centralized price determination process against the flexibility of the current decentralized approach.
Discussion
Despite reluctance to rely on a single HTA body to systematically evaluate health technologies and make coverage recommendations, there are examples of influential “HTA-like” evidence-based recommending bodies in the U.S., including the Advisory Committee on Immunization Practices (ACIP), the U.S. Preventive Services Task Force (USPSTF), and the Medicare Evidence Development & Coverage Advisory Committee (MedCAC). Although these bodies have largely been successful in their domains, the notion of conducting systematic HTAs in a more centralized manner to inform coverage decisions in the U.S. will likely be complicated by political resistance, particularly if economic evidence is considered as part of an assessment.
Additionally, an official HTA organization in the U.S.—even one that limits its scope to evaluating clinical and/or economic evidence and stops short of making coverage recommendations—would likely face similar challenges as the Affordable Care Act (ACA), including a polarized political environment, concerns about fiscal waste, public distrust of government interference in healthcare, and stakeholder and lobbyist opposition that complicate implementation. To win favorable public opinion, HTA processes should be transparent so that they avoid accusations of subjectivity or government-sanctioned “death panels.” Although cost must be considered in resource allocation decisions, particularly if the aim is to tie prices to value, effective communication and public relations to anticipate controversies are key to retaining bipartisan support for a sustainable HTA environment in the U.S.
Although many healthcare systems around the world rely on both public and private payers, most HTA and pricing processes discussed here apply to the public option in these countries. Formally using results generated by an HTA body in drug pricing decisions in the U.S. would also require a determination of which plans would be impacted. One option would be to offer tiered plans (such as gold, silver, bronze) that use different cost-effectiveness thresholds for their coverage decisions. Another possibility would involve creating a public option for drug coverage in the U.S., whether prices are determined through negotiation or price setting based on HTA information. Irrespective of how the U.S. might implement a process that directly links HTA to pricing, policymakers will need to consider potential spillover effects on payers that do not participate and how these changes will impact patients, the healthcare system, and society at large.
Conclusion
The U.S. healthcare system is complex and comprised of both public and private payers. Addressing the cost of healthcare, including health technologies, remains a high priority, yet legislation aimed at reducing prices has been largely ineffective. While the U.S. should strive to develop novel solutions for setting healthcare prices efficiently while encouraging future innovation, the creation of a formal HTA body may be a way to foster impartial evidence generation related to the value of health technologies and serve as a complement to other policy solutions.
The USC Schaeffer Center-Aspen Institute Advisory Panel on Health Technology Assessment in the U.S. is hosted by Dana P. Goldman and Ruth J. Katz. Darius Lakdwalla, Peter J. Neumann, and Gail R. Wilensky are serving as co-chairs of the panel. The following people participated in the first panel meeting in October 2019: Alan Balch, PhD, Louis Garrison, PhD, Adrian Griffin, MSc, Margaret A. Hamburg, MD, Joel W. Hay, PhD, Zeba M. Khan, PhD, Finn BØrlum Kristensen, MD, PhD, Samuel Nussbaum, MD, Daniel A. Ollendorf, PhD, William Padula, PhD, Charles E. Phelps, PhD, Mark Sculpher, PhD, and Martin Zagari, MD. These panelists were not involved in drafting or reviewing this paper.
Footnotes
[i] Despite lacking a single national HTA program (i.e., something like The National Institute for Health and Care Excellence (NICE) in the U.K.), several organizations in the U.S. engage in HTA activities, including the Department of Veterans’ Affairs[8] and the Centers for Disease Control and Prevention.[9]
[ii] A comprehensive review of all HTA efforts in the U.S., both public and private, is outside the scope of this paper. Readers who are interested in additional
historical context should read publications by Luce and Cohen (2009), Sullivan et al. (2009), Trosman et al. (2011), and Wong (2014).[8,10-12]
[iii] Although the OTA was an official national HTA organization, it did not conduct assessments of all newly approved technologies as is done by HTA bodies in other countries. Moreover, the OTA also evaluated issues in areas outside of health, including the environment and transportation.
[iv] The committees are the California Technology Assessment Forum (CTAF), the Midwest Comparative Effectiveness Advisory Council (Midwest CEPAC), and the New England CEPAC.
[v] Cost-effectiveness analyses (CEA) are a key input in many HTAs and provide a way to incorporate economic information in the assessment. CEA methods are well-developed and provide a framework for comparing the relative value of health interventions. The general principles and best practices for CEA and value assessment have been discussed previously by the Second Panel on Cost-Effectiveness[17] and the ISPOR Special Task Force,[18] and are not the primary focus of the current advisory panel.
[vi] Economic evidence is usually incorporated into HTAs through CEA, although other methodologies might be used to assess whether the proposed price for a health technology or intervention is reasonable given the health benefits it provides. Additional economic factors, such as budget impact may also be considered, although these factors are separate from CEA.
[vii] Linking HTA to pricing is more straightforward for drugs since they have a larger body of clinical data (including efficacy and safety) prior to their market introduction. However, this discussion could extend to other types of medical care, including devices, procedures, and services.
[viii] While the government would have a relatively strong negotiating position and could eliminate the role of middlemen, government prices could still be inefficient if they are artificially low or not linked to value.
[ix] Government-mandated (compulsory) marketing of a product following failed price negotiations is another mechanism for ensuring drug access, but runs counter to free-market principles.
[x] Current examples of potential legislation aimed at regulating drug prices include the Lower Drug Costs Now Act (H.R.3) and the Prescription Drug Price Relief Act (S.102).[24,25]
References
1. Papanicolas, I., L.R. Woskie, and A.K. Jha, Health Care Spending in the United States and Other High-Income Countries. JAMA, 2018. 319(10): p. 1024-1039.
2. Anderson, G.F., et al., It’s The Prices, Stupid: Why The United States Is So Different From Other Countries. Health Aff, 2003. 22(3): p. 89-105.
3. Anderson, G.F., P. Hussey, and V. Petrosyan, It’s Still The Prices, Stupid: Why The US Spends So Much On Health Care, And A Tribute To Uwe Reinhardt. Health Aff, 2019. 38(1): p. 87-95.
4. Dieleman, J.L., et al., Factors Associated With Increases in US Health Care Spending, 1996-2013. JAMA 2017. 318(17): p. 1668-1678.
5. Sood, N., et al., The Effect of Regulation on Pharmaceutical Revenues: Experience in Nineteen Countries. Health Aff, 2009. 28(1): p. w125-37.
6. Yu, N.L., P. Atteberry, and P.B. Bach. Spending On Prescription Drugs In The US: Where Does All The Money Go? Health Affairs Blog 2018; Available from: https://www.healthaffairs.org/do/10.1377/hblog20180726.670593/full/.
7. Centers for Medicare & Medicaid Services. NHE Fact Sheet. 2019; Available from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet.html.
8. Luce, B. and R.S. Cohen, Health Technology Assessment in the United States. Int J Technol Assess Health Care, 2009. 25 Suppl 1: p. 33-41.
9. Drummond, M.F., et al., Key Principles for the Improved Conduct of Health Technology Assessments for Resource Allocation Decisions. Int J Technol Assess Health Care, 2008. 24(3): p. 244-258.
10. Sullivan, S.D., et al., Health Technology Assessment in Health‐Care Decisions in the United States. Value Health, 2009. 12: p. S39-S44.
11. Trosman, J.R., S.L. Van Bebber, and K.A. Phillips, Health Technology Assessment and Private Payers’ Coverage of Personalized Medicine. J Oncol Pract, 2011. 7(3S): p. 18s-24s.
12. Wong, J., The History of Technology Assessment and Comparative Effectiveness Research for Drugs and Medical Devices and the Role of the Federal Government. Biotechnol Law Rep, 2014. 33(6): p. 221-248.
13. Sadowski, J., Office of Technology Assessment: History, Implementation, and Participatory Critique. Technol Soc 2015. 42(C): p. 9-20.
14. Patient-Centered Outcomes Research Institute, Compilation of Patient Protection and Affordable Care Act: Extracted Sections Concerning Patient-Centered Outcomes Research and the Authorization of the Patient-Centered Outcomes Research Institute (PCORI). 2010. 2017.
15. Patient-Centered Outcomes Research Institute. PCORI Statement on Congressional Reauthorization of Funding. 2019; Available from: https://www.pcori.org/news-release/pcori-statement-congressional-reauthorization-funding.
16. Institute for Clinical and Economic Review. ICER Launches New Drug Assessment Program with $5.2 Million Award from the Laura and John Arnold Foundation. 2015; Available from: https://icer-review.org/announcements/icer-ljaf-drug-assessment-announcement/.
17. Sanders, G.D., et al., Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA, 2016. 316(10): p. 1093-1103.
18. Garrison Jr, L.P., et al., A Health Economics Approach to US Value Assessment Frameworks—Summary and Recommendations of the ISPOR Special Task Force Report [7]. Value Health, 2018. 21(2): p. 161-165.
19. Kolber, M.S., Opacity and Cost Effectiveness Analysis in Medicare Coverage Decisions: Health Policy Encounters Administrative Law. Food Drug Law J, 2009. 64: p. 515-530.
20. Neumann, P.J., Why Don’t Americans Use Cost-Effectiveness Analysis. Am J Manag Care, 2004. 10(5): p. 308-312.
21. Fox, J., Medicare Should, but Cannot, Consider Cost: Legal Impediments to a Sound Policy. Buffalo Law Rev, 2005. 53: p. 577.
22. Chambers, J.D., M.J. Cangelosi, and P.J. Neumann, Medicare’s Use of Cost-Effectiveness Analysis for Prevention (but Not for Treatment). Health Policy, 2015. 119(2): p. 156-163.
23. Mulcahy, A. The Promise and Peril of Offshoring Prescription Drug Pricing. 2019; Available from: https://thehill.com/opinion/healthcare/461406-the-promise-and-peril-of-offshoring-prescription-drug-pricing.
24. Elijah E. Cummings Lower Drug Costs Now Act, in H.R.3, 116th Congress. 2019-2020.
25. Prescription Drug Price Relief Act of 2019, in S.102, 116th Congress. 2019-2020.

You must be logged in to post a comment.