Editor’s Note: This piece originally appeared on Health Affairs blog.
About 10 percent of all healthcare spending is on prescription drugs, and the spending is growing at a rapid rate. This has prompted calls for government intervention to regulate drug prices or otherwise control their rapid increase. But any intervention should be predicated on a clear understanding of the economic forces that drive price increases, and the parties responsible for them.
Much attention has focused on the average wholesale or “list” price set by manufacturers prior to discounts. These prices have been increasing — the average list price of branded drugs rose 12.4 percent in 2015, and has increased 10 percent or more annually since 2012. Yet list prices rarely represent what manufacturers are paid for drugs, as they are routinely discounted and rebates paid to various parties in the distribution system.
Net prices—which include all discounts and rebates—have also risen, albeit more slowly — 2.8 percent in 2015. Yet even the net price that manufacturers receive does not fully represent what patients pay, with the difference being allocated among other stakeholders in the drug distribution chain, including insurers, pharmacy benefit managers (PBMs), pharmacies, and wholesalers. Contracts among these players govern the exchange of goods (drugs) or services (such as logistics or claims administration) for various fees, discounts, rebates, and chargebacks. Such arrangements are typically privately negotiated and undisclosed, making it difficult to determine precisely how large these payments are, and how they are distributed.
But publicly traded companies in the distribution system must disclose annual financial information to the U.S. Securities and Exchange Commission (SEC) and other regulatory agencies, and those reports provide some of the most reliable public information about how funds flow through the pharmaceutical distribution system. While these data are highly aggregated and permit only a high-level view of a complex system, they permit a fact-based understanding of who profits from pharmaceutical spending—and by how much—which is of great policy salience. We recently used these data to examine the flow of funds in the pharmaceutical distribution system, and have just released the results in a new study (Note 1).
Who Are The Players?
Figure 1 illustrates how pharmaceuticals are distributed in retail settings: Beneficiaries and sponsors pay premiums to a health plan, in exchange for drug coverage benefits for plan members. The health plan or self-insured employer contracts with a PBM to manage the plan’s drug benefit in exchange for fees and payments. The PBM negotiates with drug manufacturers to provide preferred formulary placement for the manufacturers’ products and processes pharmacy claims, in exchange for rebates and other fees payable to the PBM. Some of the rebates are shared with the health plan and the remaining kept as profits.
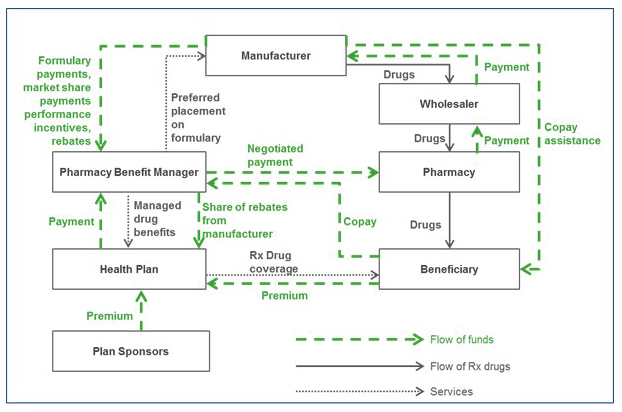
The PBM also negotiates contracts with pharmacies, including those in its network, to set reimbursements for the drugs dispensed by the pharmacy. Pharmacies in turn negotiate agreements with drug wholesalers, setting the wholesale rates at which they obtain the drugs, and wholesalers negotiate to buy drugs from manufacturers and distribute them to pharmacies.
When a covered beneficiary fills a prescription at a retail pharmacy, the pharmacy collects the beneficiary’s copayment or coinsurance and dispenses the drug from inventory. The pharmacy passes the copayment to the PBM, and the PBM pays the pharmacy the negotiated reimbursement. To re-stock inventory, the pharmacy purchases drugs from the wholesaler, who purchases them from the manufacturers.
How Does The Money Flow?
In the figure, each sector supplies goods or services to customers in exchange for payment (revenue). Some of that revenue is in turn passed from the original supplier to the next player in the chain, as payment for the raw goods and services needed to produce the product, called “cost of goods sold.” The remaining funds, or gross profit, are captured in the sector, and may be used in several ways, including to fund research and development (R&D), marketing, general and administrative activities (SG&A), or provide a return to owners (net earnings or profits).
Gross profits are the difference between revenues received and costs of goods sold; for example, gross profits for wholesalers are revenues received primarily from pharmacies less payments made primarily to manufacturers. Some of these gross profits are spent on other business expenses such as marketing, R&D, depreciation, interest, and taxes. What remains after subtracting these expenses is net profits, which accrue to the shareholder owners of the firm.
How Much Are People Making?
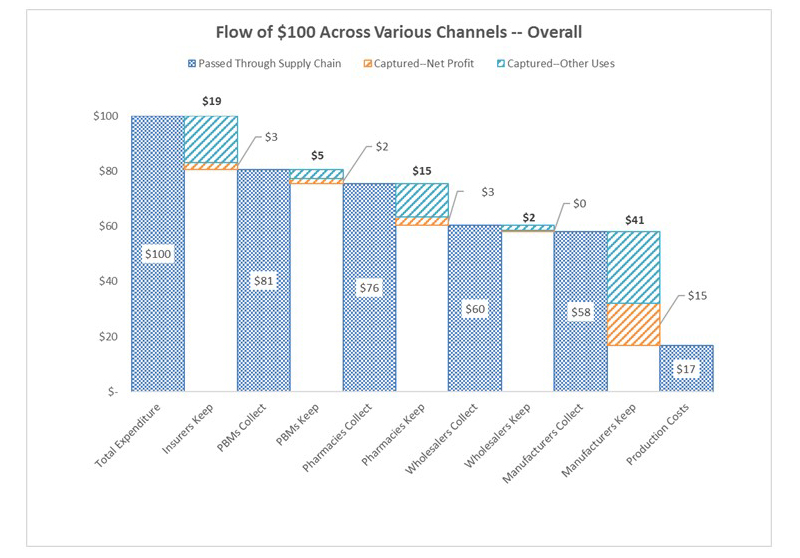
Based on the results of our study, we explore a hypothetical scenario in which $100 is spent on prescription drugs acquired at a retail pharmacy using commercial insurance, using estimates of gross and net margins as a fraction of net revenues from companies’ 2015 SEC filings. Of a $100 expenditure on pharmaceuticals by consumers, roughly $58 goes to the manufacturer and $41 is captured by intermediaries. (Numbers do not sum to 100 due to rounding.) Of the $58 received by the manufacturer, $17 is spent on drug production and the remaining $41 is spent on other expenditures (such as marketing and R&D) or kept as net profit. Total net profit on a $100 expenditure is $23, of which $15 goes to manufacturers and the remaining $8 goes to intermediaries including $3 to insurers, $3 to pharmacies, and $2 to PBMs.
Of course, the flow of funds differs between branded and generic market segments. While manufacturers make about three times the gross profits on branded vs. generic drugs ($58 vs. $18, consistent with the market exclusivity granted to patented drugs), other segments make much more on generic expenditures: PBMs make four times as much on generic drugs compared to brands, while wholesalers make 11 times as much, and pharmacies almost 12 times as much, $32 compared to $3.
Are The Returns ‘Excessive’?
A natural question is whether some of the stakeholders are making excessive returns. Market concentration is an important indicator of companies’ ability to earn extraordinary returns, and several segments in the US pharmaceutical distribution system are highly concentrated. The top three PBMs, wholesalers, and retailers account for 85 percent, 66 percent, and 49 percent of their markets, respectively. This market power manifests in practices that likely raise consumer prices. For example, pharmacies charge widely varying prices for exactly the same product, and the uninsured often pay higher prices than insured consumers.
Some claim market concentration is good for consumers. For example, PBMs argue that their large size gives them bargaining power to negotiate lower prices for their clients. Less clear is the extent to which these savings are passed on to health plans and consumers. PBMs carefully guard information about the size of negotiated rebates and discounts, which may enhance their ability to negotiate lower prices, but also masks whether they are indeed lowering the prices paid by patients and insurers as claimed. In fact, recently proposed legislation intended to lower drug prices would require PBMs to disclose rebates and the share passed on to health plans.
While the market for manufacturers is less concentrated, patents and market exclusivity for many products confer a complete monopoly for those drugs. We find that brand manufacturers’ net profit margins are high compared to other manufacturing industries, suggesting excessive returns. However, returns for manufacturers, particularly branded drugs, must compensate for the risk taken in drug development. Other manufacturing industries where intellectual property protections are important, including software, semiconductors, and computer equipment also demonstrate higher margins.
Nonetheless, manufacturers also engage in some questionable practices. Manufacturers of branded drugs facing competition from new generic entrants may fund copay assistance programs that reduce patients’ out-of-pocket costs, dissuading them from switching to cheaper generics and circumventing insurer formularies designed to encourage such switching. Manufacturers have also been scrutinized for off-label promotion of drugs and anticompetitive pricing practices, including the ongoing investigation surrounding rising insulin prices.
Insurer practices also have competing effects on consumer drug costs. On one hand, they exert downward pressure on prices when they place the products of manufacturers who refuse to lower prices in higher cost-sharing or copayment tiers. But the resulting high out-of-pocket costs burden consumers who need these expensive drugs, and lower adherence. The practice also doubly penalizes those who do not respond to less expensive therapies.
Sometimes consumers pay more for a prescription than the insurer’s cost of acquiring the drug — a common experience in high-deductible health plans. Members who have not exhausted their deductible pay out-of-pocket the full average wholesale price for drugs, although the plan acquires them at a discount and collects the associated manufacturer rebate. For generic drugs, consumers’ copays may exceed the cost of the drug. By contrast, patients would not likely tolerate paying an office visit copayment that exceeded their physician’s reimbursement.
What Is The Bottom Line?
According to our study, for every $100 spent for retail prescription drugs in the US, about $17 compensates for direct production costs, $41 accrues to the manufacturer, and $41 accrues to intermediaries in the distribution system: wholesalers, pharmacies, pharmacy benefit managers, and insurers. (Numbers do not sum to 100 due to rounding.) These allocations differ depending on whether the drug is generic or branded, and, taken together, illuminate opportunities to reduce total drug spending.
Put another way, more than $1 out every $5 spent on prescription drugs goes towards profits in the pharmaceutical distribution system. While we cannot say definitively whether any sectors are making excessive profits, our results are consistent with profit-making behavior on the part of all sectors. Greater scrutiny of their pricing policies and more competition throughout the distribution system is warranted. The question of what drives high intermediary profits on generic products is especially interesting.
Any policy effort to control drug prices through regulation or other means would benefit from greater transparency and granularity in reporting of each sector’s financials, which is required to fully understand the dynamics in specific market segments. In this regard, recent initiatives by several pharmaceutical manufacturers to provide more price transparency are welcome. In any case, efforts to control drug costs should focus on the rents enjoyed by all players in the distribution system.
Note 1
Support for the study was provided by the Schaeffer Center for Health Policy & Economics at the University of Southern California and by Amgen through a contract with Precision Health Economics. The views expressed in this post and in the study are those of the authors and do not represent the views of the funders, who had no role in the preparation of either. Goldman is a co-founder of Precision Health Economics and holds equity in its parent company; he is also the Leonard D. Schaeffer Chair and Director of the Schaeffer Center. Shih is a research economist at Precision Health Economics. Sood and Van Nuys have served as consultants to PHE; in addition, Sood is Director of Research at the Schaffer Cener, and Van Nuys is Executive Director of the Value of Life Sciences Innovation Project there.
Authors’ Note
This research was supported by Amgen under contract with Precision Health Economics, a health care consultancy. Goldman is a co-founder of Precision Health Economics and Sood and Van Nuys are consultants. The authors are solely responsible for the design and conduct of the study and the sponsor had no control over editorial content.
Copyright ©2015 Health Affairs by Project HOPE – The People-to-People Health Foundation, Inc.
You must be logged in to post a comment.