For the vast majority of cancer drugs experiencing shortages over a seven-year period, researchers found no statistically significant effect of shortages on chemotherapy treatment.
“These findings are surprising in light of the substantial media and policy attention that the cancer drug shortage problem has garnered,” said Mireille Jacobson, study coauthor and associate professor of gerontology at the USC Leonard Davis School and the Schaeffer Center for Health Policy & Economics.
Although the proportion of patients receiving treatment declined for six drugs, including fluorouracil, doxorubicin, and cytarabine, the use of 32 other cancer drugs was unaffected or even increased during shortage episodes. Likewise, dosages declined for only a few drugs during shortages. The results were published in Clinical Pharmacology & Therapeutics.
The authors noted that while the shortages may not have been noticeable from a treatment perspective, they may have come at personnel and psychic costs not measured by the study. “This study only measured one dimension of oncology drug shortages: the effect on utilization,” said Jacobson.
Increasing Oncology Drug Shortages Raised Alarms
The number of prescription drug shortages in the United States increased from 71 in 2005 to 255 in 2011, and shortages of oncology drugs more than doubled during that time period. Oncology drug shortages in particular have generated substantial attention from the media and policymakers because of concerns about treatment gaps and inadequate dosing. For cancers in which certain treatments are clinically preferred or no alternative exists, drug shortages may be life-threatening.
Until now, no studies have systematically analyzed the consequences of shortages nationwide. Using data from the Surveillance, Epidemiology, and End Results (SEER) cancer registries linked with Medicare claims, Jacobson and colleague Abby Alpert with the University of Pennsylvania set out to provide a more general analysis of the impact of oncology drug shortages on outpatient chemotherapy treatment.
Millions of Claims, Thousands of Patients, Null Impact on Chemotherapy
Jacobson and Alpert studied more than 2.4 million monthly claims for chemotherapy treatment administered in a physician’s office or outpatient hospital or clinic setting over a seven-year period. The claims were from 182,470 Medicare beneficiaries newly diagnosed with seven common cancers, including breast, colorectal, leukemia, lung, lymphoma, ovarian and pancreatic cancer.
The newly diagnosed sample represented 60 percent of total chemotherapy claims in the SEER-Medicare data. One in three chemotherapy claims for the newly diagnosed were for drugs that were on shortage during the study period.
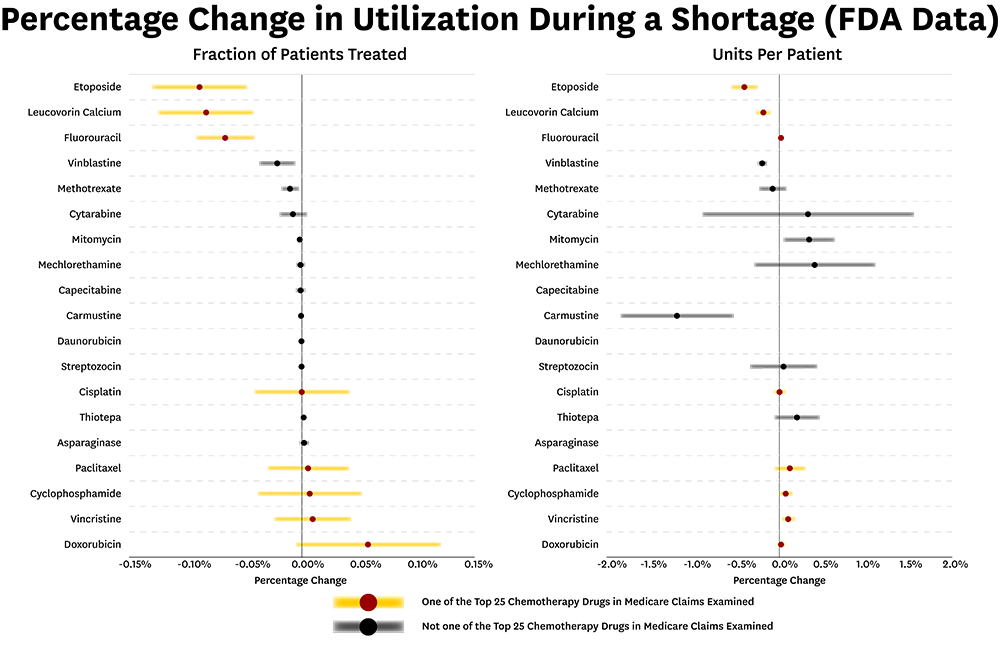
The authors found little impact on outpatient chemotherapy treatment for the majority of oncology drugs identified as experiencing shortages between 2004 and 2011. Even among the most commonly used chemotherapy drugs— those ranked in the top 25 prescribed drugs in the sample—researchers found almost no change in treatment during shortages, including minimal effect on dosing volume.
The authors found the portion of patients receiving treatments declined for six drugs, including generic drugs that have been on the market for decades.
“It seems that when some drugs become older and very low cost, there is not as much investment in making sure they are available,” said Jacobson.
Possible Explanations
Researchers say one explanation for the findings is that most oncology drug shortages have been effectively managed to minimize the effects on treatment. “Although a shortage may reflect a complete disruption of production, providers may be able to draw from existing inventories, purchase from providers who have higher projected inventory, or stockpile in advance if a shortage is anticipated,” said Jacobson.
A second explanation is that rapid or timely production increases, sometimes at the request of the FDA, could have mitigated the impact of shortages on chemotherapy treatment. In some cases, temporary FDA allowance of importation of unapproved foreign versions of scarce drugs may have further eased the effects of shortages.
Next, the authors say the database considered the gold standard for dating shortages (UUDIS) may be capturing many shortages that would not be expected to impact treatment. The database includes on its shortage list any supply disruption that affects “how a pharmacy prepares or dispenses a drug product or that influences patient care when prescribers must use an alternative agent.”
For example, if a larger vial size of the drug were unavailable, this would be classified as a shortage even if smaller package sizes were in supply and met patient demand. Although this type of supply disruption might require additional pharmacy resources, its impact on treatment might be minimal. It is unclear how many of the UUDIS shortages fit this description, however, the findings of minimal shortage impacts suggest that many may belong in this category.
Finally, researchers say the findings may be due to SEER-Medicare data limitations. The large sample size captures few nonelderly patients with cancer, and therefore may understate the effect of shortages on drugs, such as mechlorethamine, used to treat cancers that primarily affect younger populations. In addition, since SEER covers only a limited set of geographic areas, the data may miss regions that are harder hit by shortage.
How can Drug Shortages Be Mitigated?
“A key challenge moving forward is how to separately identify those cases that will result in clinically relevant shortages from those that are relatively easy to manage,” said Jacobson. “A discussion of the intended use for, and limitations of the UUDIS and the FDA drug shortage lists would improve decision making by policymakers and physicians and the public discussion of drug shortages.”
The authors say alternative approaches to tracking shortages and measuring their effects on treatment may be warranted to facilitate the early identification and mitigation of clinically relevant shortages.
The study was supported by the National Cancer Institute grant R21 CA173047 and the Agency for Healthcare Research and Quality grant 1R01HS022741- 01A1.
You must be logged in to post a comment.