Bedsores—also known as pressure injuries—are the fastest rising hospital-acquired condition, according to the U.S. Agency for Healthcare Research in Quality, and as a result have become the second most common reason for medical malpractice suits in the United States.
Although most hospital-acquired pressure injuries are reasonably preventable, approximately 2.5 million individuals in the United States develop a pressure injury in acute care facilities every year, and 60,000 die. The total annual cost for U.S. health systems to manage the acute needs of patients’ pressure injuries during hospitalization exceeds $26 billion, and yet pressure injuries have received relatively little attention as a public health crisis.
Researchers from USC, Johns Hopkins University and University Hospitals Cleveland Medical Center collaborated to leverage machine-learning techniques to develop a new model to predict future risk of pressure injuries and better direct labor-intensive patient care.
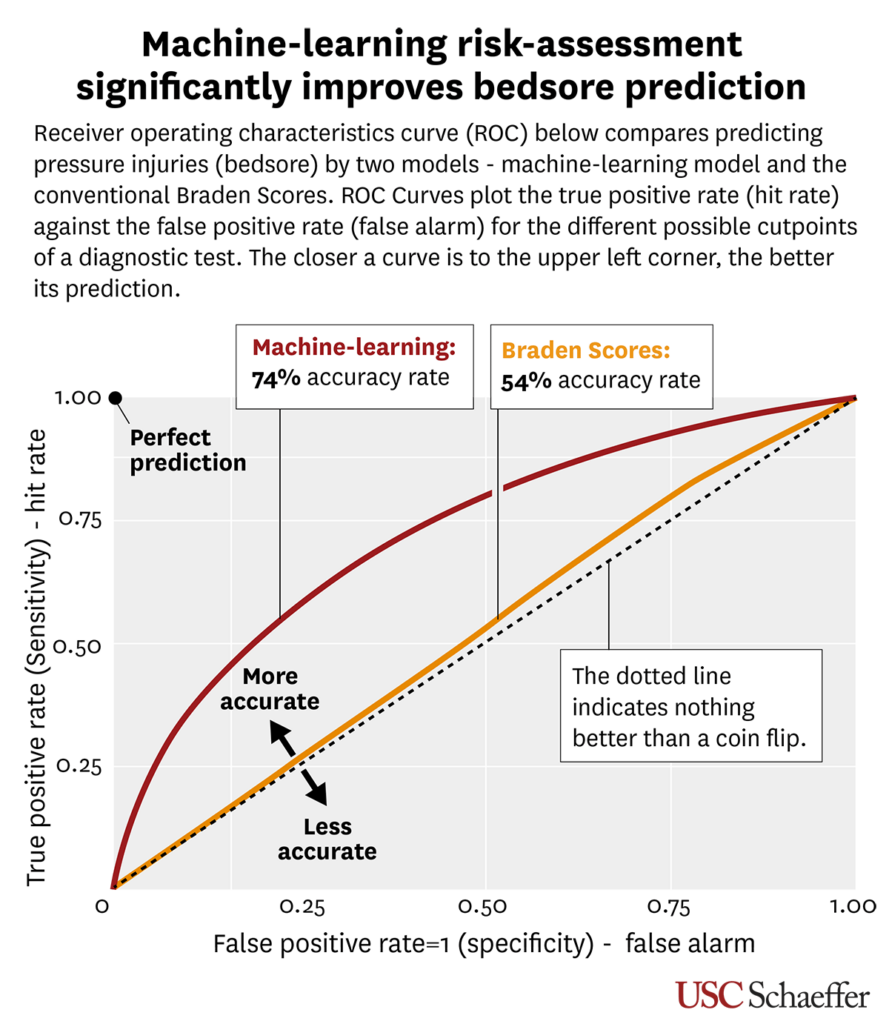
Published today in BMJ Open, the new risk-assessment model increases the accuracy of prediction to more than 74%—a more than 20% increase over existing methods.
Common practices and guidelines to prevent pressure injuries are time-consuming and taxing on nurses at the bedside. The industry-standard tool for predicting the risk of pressure injury, the paper-based Braden Scale, has not changed since its origin in the 1980s and has a 54% accuracy rate, the researchers note.
Saving time, costs and lives
Predictive analytics offers the potential to alleviate some burden on nurses and frontline healthcare providers by automating part of the risk-assessment process, the researchers note. Currently, acute care providers must perform a skin check and risk assessment for pressure injury upon admission and every 12 to 24 hours thereafter, using a standardized instrument such as the Braden Scale, which primarily assesses mobility, cognition, nutrition, and incontinence management.
“Pressure injury prevention is a costly protocol to implement on a daily basis, and the existing tool for predicting pressure injuries is barely better than a coin flip,” says William Padula, assistant professor of pharmaceutical and health economics at USC Mann and a fellow at the USC Leonard D. Schaeffer Center for Health Policy & Economics. “We thought, there’s got to be a better way of doing this. The question became, could a computer do these risk assessments better than the nurses themselves at the bedside?”
The predictive algorithm developed by the team offers improved economic efficiency and substantial savings. Since a risk assessment can take anywhere from five to 15 minutes per patient, this could represent up to 250 labor hours in a single 500-bed facility per day, and between 30,000 and 90,000 labor hours per year.
“This data could help hospitals conserve resources within a critical period of patient vulnerability of hospital-acquired pressure injury that is not reimbursed by U.S. Medicare,” says Peter Pronovost, chief quality and transformation officer at University Hospitals Cleveland Medical Center, formerly director of the Armstrong Institute for Patient Safety at Johns Hopkins Hospital.
The research also fosters improvements in health equity. Existing tools don’t account for race, skin color or age. “If you can’t see bruising on a patient’s skin because they’re Black, Hispanic or Asian, then you’re not going to identify the greater risk factors they face as quickly,” Padula adds. “Machine-learning methods are not biased by what we see in the sun’s light. This allows us to improve equity of the delivery of healthcare when it comes to the prevention of these conditions that disparately affect underrepresented minorities.”
Artificial Intelligence methods
Using machine-learning methods, researchers mined the electronic health records of more than 35,000 hospitalizations over five years at two academic hospitals to analyze changes in pressure injury risk over time. They looked at variables including admission diagnostic codes, prescription drugs, lab orders and other factors most closely associated with pressure injury risk factors.
“AI-based early detection significantly outperforms standard of care. Hospitals can use this to initiate a quality-improvement program for pressure-injury prevention that improves outcomes and significantly lowers the burden on nursing from current monitoring approaches. Further, they can customize the algorithm to patient-specific variation by facility,” says Suchi Saria, John C. Malone endowed chair and AI professor at Johns Hopkins and CEO of Bayesian Health, a clinical AI platform company.
The investigators ran analytics using machine-learning techniques like random forests and neural networks to further boil down the specific weights of those variables on the changes and risk of a pressure injury case and came up with the final model. They also identified a list of prescription drugs—beta blockers, electrolytes, phosphate replacement, zinc replacement, erythropoietin stimulating agents, thiazide/diuretics, vasopressors—that change patients’ risk for pressure injury.
“This is, to our knowledge, the most advanced methodological study to our knowledge to use artificial intelligence to help better detect pressure injuries,” says David Armstrong, professor of surgery at Keck School of Medicine of USC.
This work was supported by a National Institutes of Health grant (KL2 TR001854, PI: Padula).
Sign up for Schaeffer Center news


You must be logged in to post a comment.