Key Takeaways
- The 340B Drug Pricing Program allows eligible healthcare clinics and hospitals (“covered entities”) to purchase outpatient drugs at a 20-50% discount.
- This program is unusual among federal programs in that it involves a mandatory transfer of resources from one group of private entities (manufacturers/wholesalers) to another (providers).
- Between 2000 and 2020, the number of covered entity sites participating in the 340B program increased from 8,100 to 50,000. Hospitals comprised just over 60% of sites in 2020.
- Estimated discounted purchases through the program have increased from about $4 billion per year in 2007-2009 to $38 billion in 2020.
- The 340B program faces ongoing controversy in several areas: limited program oversight, transparency in how covered entities use funds generated by the program, and the growth in participation by contract pharmacies and DSH hospitals.
Overview
The 340B Drug Pricing Program was created in 1992 and aimed at enabling certain healthcare providers, known as covered entities, “to stretch scarce federal resources to reach more eligible patients or provide more comprehensive services.”[1] As a condition of participating in the Medicaid Drug Rebate Program (MDRP),[i] drug manufacturers are required to participate in 340B, which provides discounts on outpatient drugs purchased by eligible healthcare organizations, many of which are safety-net providers treating high percentages of uninsured or low-income patients. In 2020, total sales of 340B-discounted drugs were estimated to be $38 billion, or roughly 7% of the total U.S. drug market.[2] The Health Resources and Services Administration (HRSA)—an agency within the Department of Health and Human Services— is responsible for administering the 340B program and providing oversight, including conducting audits of covered entities and manufacturers.
Initially, covered entities included disproportionate share hospitals (DSH)—which serve a “disproportionate” share of low-income Medicare or Medicaid patients—and several types of non-hospital entities (referred to as “federal grantees”): federally qualified health centers (FQHC), FQHC look-alikes, tribal/urban Indian clinics, Native Hawaiian health centers, Ryan White HIV/AIDS program grantees, and five types of specialized clinics (e.g., hemophilia treatment centers). Hospital eligibility was expanded in 2006 to include children’s hospitals affiliated with state government and again in 2010 as part of the Affordable Care Act (ACA) to include critical access hospitals (CAH), sole community hospitals, rural referral centers, and stand-alone cancer hospitals. In addition, 340B-participating hospitals with offsite outpatient clinics (“child sites”) can register those sites for 340B if they are listed as reimbursable on the hospital’s most recently filed Medicare Cost Report.[ii, 3]
Sign up for Schaeffer Center news
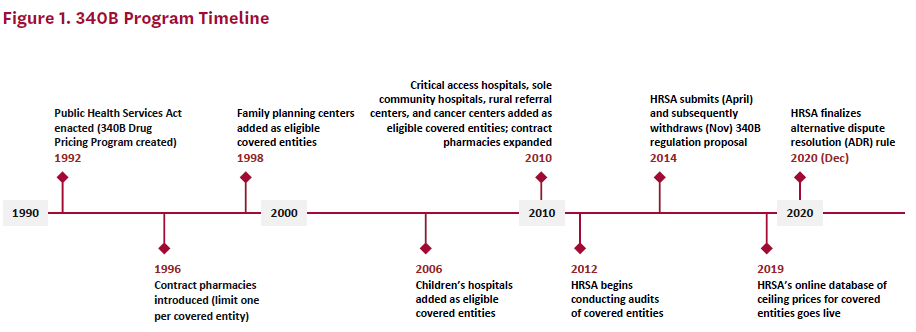
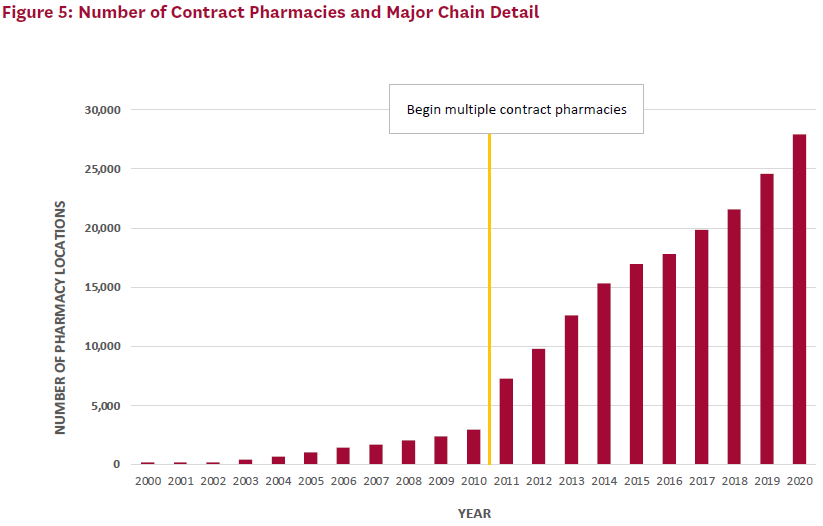
At the program’s inception, self-administered 340B-discounted drugs could only be dispensed through an in-house pharmacy. With less than 5% of covered entities using an in-house pharmacy at that time, many eligible providers could not use the 340B discount for self-administered drugs.[4] So beginning in 1996, the 340B program allowed covered entities without an in-house pharmacy to use a single external pharmacy–known as a contract pharmacy–to distribute self-administered 340B drugs to their patients. Access was further expanded in 2010 under the ACA, when covered entities were allowed to contract with an unlimited number of pharmacies to provide 340B-discounted drugs.[5] Figure 1 provides a timeline of the 340B program.
Although 340B prices are not publicly available, the program allows covered entities to acquire eligible drugs at a 20% to 50% discount.[6] Participation in the 340B program generates revenues for covered entities if insurance reimbursements exceed 340B acquisition costs. While the 340B legislation does not specify how providers should use 340B proceeds, certain covered entities are required to use 340B revenue according to their grant requirements, which usually specify that they be used to advance the goal of providing high-quality, affordable care to underserved populations.[7] As an example, passing 340B discounts on to patients would count toward this reinvestment requirement, but covered entities can choose to reinvest the funds in other ways, including offering expanded services. By contrast, participating hospitals are not required to use their 340B revenues in any particular way.[7, 8]
Which Drugs and Patients Are Eligible for 340B Discounts?
With a few exceptions, covered entities can purchase nearly all self- or physician-administered drugs dispensed in the outpatient setting at the 340B discounted price. Vaccines are not eligible for the 340B discount, and orphan drugs—defined as those treating a rare disease or condition—are excluded from 340B discounts for covered entities that became eligible for 340B under the ACA. Additionally, drugs obtained by DSH, cancer, and children’s hospitals through group purchasing organizations or arrangements are excluded from 340B discounts.
Covered entities may only dispense drugs purchased with 340B discounts to “eligible patients.” Although there are no income- or insurance-based requirements for patient eligibility, HRSA developed a patient definition to prevent covered entities from dispensing drugs purchased with 340B discounts to patients who do not receive outpatient services at the covered entity. Specifically, patients must have an established relationship with the covered entity, receive health care services from a health care professional employed by the covered entity,[iii] and receive a healthcare service or range of services consistent with the service(s)for which grant funding or FQHC look-alike status has been provided to the entity.[iv, 9] The patient definition precludes individuals who only receive prescription drugs from the covered entity (but no other health care services) from receiving drugs purchased with 340B discounts.
When drugs purchased with 340B discounts are dispensed to ineligible patients–for example, an inpatient at a hospital or a patient who is written a prescription by a provider that is not employed by the covered entity—this is referred to as “drug diversion.” Diversion risk is higher in hospitals because they see both inpatients (whose drugs are not eligible for 340B discounts) and outpatients (whose drugs are.) Hospitals may also have free-standing clinics that are not registered for 340B; as such, they cannot dispense drugs purchased with 340B discounts despite their affiliation with a covered entity. Diversion risk is also higher with contract pharmacies because they dispense drugs to both patients of covered entities (whose outpatient drugs are eligible for 340B discounts) and patients of non-340B-covered entities (whose drugs are not.) To prevent the diversion of drugs acquired with 340B discounts to ineligible patients, covered entities and contract pharmacies may either keep separate physical inventories for drugs purchased with and without 340B discounts, or they may track the inventories virtually. While the full extent of drug diversion in the 340B program is unknown, across the 1,242 audits conducted between 2012 and September 2020, HRSA reported 546 diversion-related findings.[10]
How Are Discounted 340B Drug Prices Determined?
The maximum amount a manufacturer can charge a covered entity for the purchase of a 340B covered drug is called the “340B ceiling price,” and is based on the average manufacturer price (AMP). In the ceiling price calculation, the AMP is reduced by the Unit Rebate Amount (URA), which is based on the Medicaid “best price,” defined as the lowest available price to any wholesaler, retailer, or provider, excluding certain government programs, such as the health program for veterans.[11] Manufacturers submit AMP and URA to the Centers for Medicare and Medicaid Services (CMS) for quarterly MDRP reporting, and these are used to calculate 340B ceiling prices. For branded drugs,[v] the URA is the larger of 23.1% of AMP or the difference between AMP and the Medicaid best price. If the calculated ceiling price is zero, manufacturers charge a penny for the drug.[12] Manufacturers may also offer sub-ceiling discounts, and covered entities can choose to work with the Prime Vendor Program (created in 2004 through a contract with HRSA to support the 340B Program[13]) which may negotiate better prices on behalf of participating entities.
How is the 340B Program Administered?
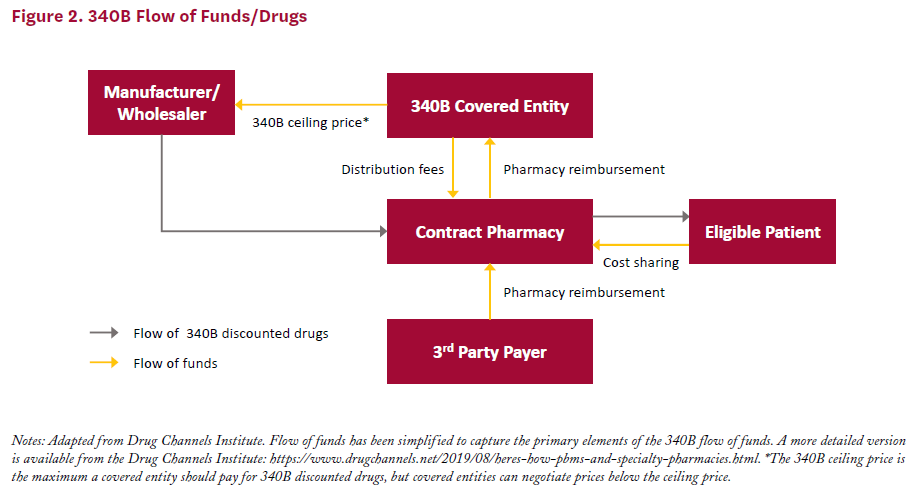
Covered entities obtain drugs at 340B prices either through a direct purchase or through back-end discounts. Under the direct purchase option, covered entities buy drugs from manufacturers or wholesalers and pay 340B discounted prices up front. Alternatively, covered entities can purchase drugs at full price through a vendor, then receive manufacturer discounts on the back end for any amount paid over the 340B ceiling price. Figure 2 provides a visual representation of the flow of funds in 340B.
Trends in 340B Participation and Program Size
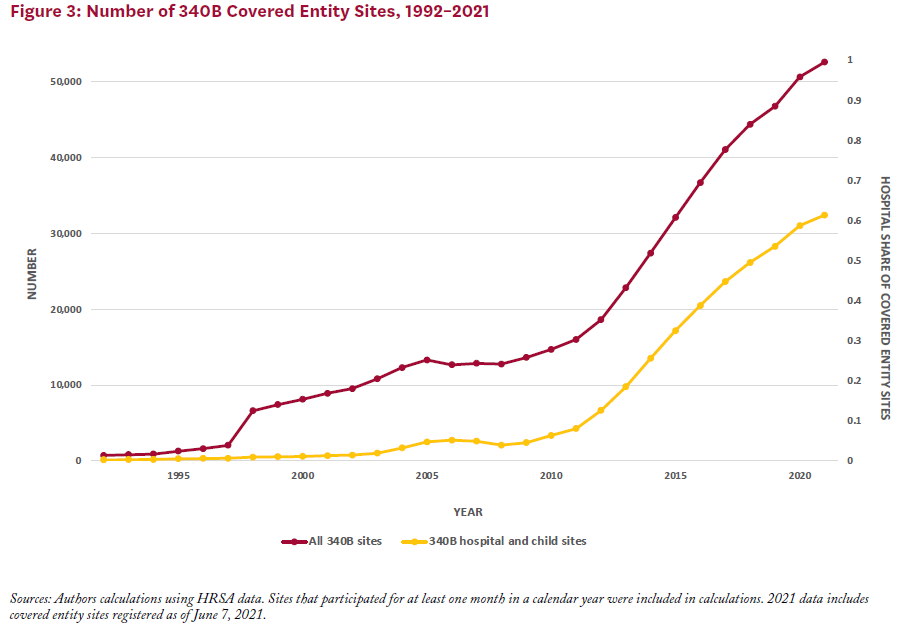
Between 2000 and 2020, the number of covered entity sites participating in the 340B program increased from just over 8,100 to 50,000 (Figure 3). The number of covered entities grew more rapidly following the expansion of covered entity types in 2010, driven primarily by participation among CAHs. Prior to 2004, hospitals represented less than 10% of covered entity sites (including child sites); by 2020 they made up just over 60% of covered entity sites.
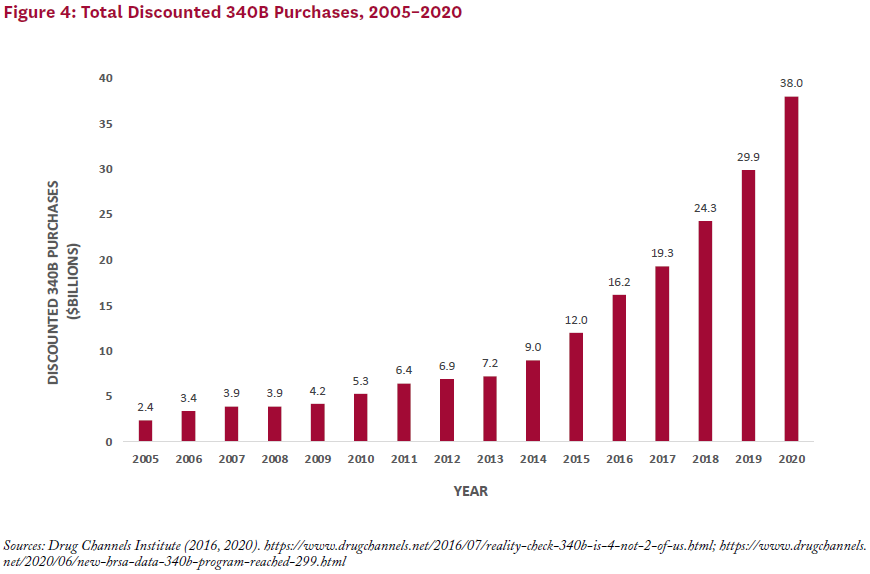
Program sales have grown along with the number of covered entities, with total estimated discounted purchases for the 340B program rising from about $4 billion per year in 2007–2009 to $38 billion in 2020—or roughly 7% of the total U.S. drug market (see Figure 4).[2, 14]
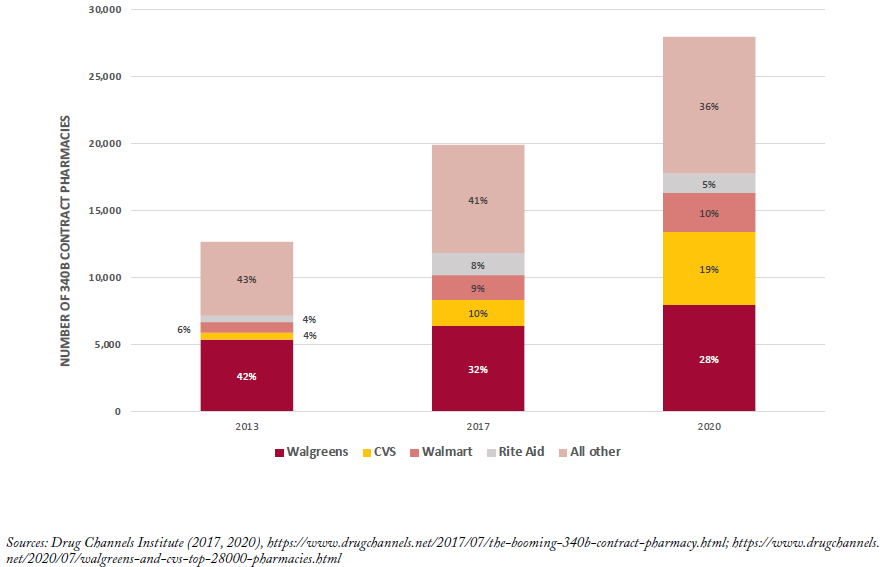
The number of contract pharmacy sites has also increased over time (Figure 5). Of note, when covered entities were first allowed to use multiple contract pharmacies, the total number of locations increased by nearly 150%. Large retail pharmacy chains—Walgreens, CVS, Walmart, and Rite Aid— are disproportionately represented among contract pharmacies and together accounted for just over 60% of locations in 2020.
Implications of the 304B Program
Covered entities and their advocacy groups argue that the 340B program has enabled them to stretch scarce federal resources and help provide care for vulnerable populations. However, because the program lacks transparency and reporting requirements around the discounted prices paid by covered entities, their program savings, or how those savings are used, it faces ongoing scrutiny over whether it aligns with the legislation’s original intent.
For example, the National Association of Community Health Centers claims the 340B program has helped many FQHCs—which provide healthcare to 1 in 11 people in the U.S.—to remain open, provide care to more patients, or expand services.[15] A subset of hemophilia treatment centers reported they use 340B savings to finance important elements of care coordination (e. g., nurses, social workers, telemedicine) that are not reimbursed by third party payers.[16] Ryan White clinics provide care for half of the population with HIV/AIDS, and RWC-340B claims that 340B drug savings have enabled them to provide free or low-cost care as well as support services such as housing and food assistance.[17] Hospital organizations such as 340B Health argue 340B savings are particularly important for hospitals in rural areas (which often do not have enough residents to support a hospital) or that provide care for underserved populations. Seventy-five percent of CAHs—which have fewer than 25 beds and are located more than 35 miles from another hospital—report 340B savings help them remain open.[18]
Sign up for Schaeffer Center news
But such claims by stakeholders and other interested parties should be weighed alongside broader quantitative analyses by unbiased observers. While the lack of publicly available program data makes such analyses difficult, some have shown 340B program benefits using more robust empirical approaches. For example, one study found HIV/AIDS patients who receive their drugs from a 340B program have higher medication adherence compared with patients who do not.[19] A recent study that surveyed pharmacy directors at 340B and non-340B hospitals found 340B hospitals provide more medication access services such as prior authorization assistance or free or discounted medications and were also more likely to provide outpatient services for drug treatment or HIV/ AIDS.[20] Finally, one study estimated that hospital 340B profits from Medicare were relatively small compared with overall operating budgets and uncompensated costs–on average 0.3% and 9.4%, respectively.[21]
Despite claims and evidence highlighting the benefits of the 340B program, several issues with the 340B program remain the focus of ongoing controversy. For example, the 340B program is unusual among federal programs in that it involves a mandatory transfer of resources from one group of private entities (drug manufacturers and wholesalers) to another group of private entities (providers), and its founding legislation provided little guidance or restrictions on how covered entities should utilize the funds the program generates.[22] Its rapid growth and lack of oversight has also drawn scrutiny. The 340B program has been examined regularly by the Government Accountability Office (GAO) and the Department of Health and Human Services Office of Inspector General (OIG), and their reports have highlighted several issues with the program, including limited oversight, lack of transparency, concerns stemming from DSH hospitals and contract pharmacies, and duplicate discounts.
Limited Regulatory Authority Given to HRSA The 340B program is administered by HRSA’s Office of Pharmacy Affairs, but HRSA has limited regulatory authority over 340B. However, HRSA has rulemaking authority in three areas: 340B ceiling price calculations, manufacturer overcharge civil monetary penalties, and alternative dispute resolution (ADR). The ACA also included provisions that would allow HRSA to impose sanctions for covered entities that are not in compliance with program requirements.[23] However, despite having these authorities since the inception of the 340B program, HRSA has only recently established final rules in these domains. Additionally, HRSA does not have authority over other areas of 340B, including making rules to modify or clarify the eligible patient definition7 or enforcing its own guidance that allows covered entities to use unlimited contract pharmacies.[24]
While the success of the 340B program hinges on covered entities being able to take advantage of 340B discounts, in practice this has not been straightforward. Historically, HRSA calculated ceiling prices for oversight purposes, but covered entities could not access the information because it was based on proprietary manufacturer data. Consequently, covered entities could be overcharged for 340B drugs. This issue was flagged as early as 2005 by the OIG, and HHS was directed to develop a ceiling price database for covered entities in 2010 as part of the ACA.[25] It took nearly a decade for the issue to be resolved, but as of April 2019, covered entities can access ceiling prices through a HRSA database.
Along with the creation of the ceiling price database, the regulation covering civil monetary penalties was delayed nearly a decade and became effective in January 2019.[26] Prior to the final rule, HRSA did not issue civil monetary penalties partly because covered entities lacked ceiling price data: Since covered entities did not know whether they were being overcharged, it was difficult for them to know when to seek penalties. Lack of monetary penalties or sanctions for covered entities also stemmed from HRSA’s reliance on self-policing by covered entities to ensure compliance with drug diversion and duplicate discount requirements (discussed below).[6] Even after HRSA introduced formal audits, their resources were limited; fewer than 200 audits have been conducted annually since 2011.[27] Although audits are limited to a small share of covered entities, noncompliance is potentially high: Of the 1,240 audits conducted between 2012 and 2019, roughly 75% resulted in at least one finding of noncompliance.[10] Furthermore, covered entities have not been penalized for noncompliance found during those audits and HRSA has primarily relied on self-attestation by covered entities to ensure corrective actions were taken following an audit.[27]
HRSA has had regulatory authority for alternative dispute resolution (ADR) since 340B was created. HHS attempted to finalize the creation of an ADR process in December 2020, however, legal challenges may further delay implementation.[28, 29, 30] In its current proposed form, the ADR regulation will enable HRSA to create an ADR Board consisting of at least six appointed members from HHS, HRSA, and the HHS Office of General Council. The ADR process will provide a means to resolve claims with damages greater than $25,000 by 1) covered entities who have been overcharged for drugs by manufacturers and 2) manufacturers who have audited a covered entity and found evidence of drug diversion or duplicate discounts. The rule allows for consolidated claims by covered entities or manufacturers. Claims will be reviewed by 3-member ADR Panels selected from the ADR Board. The ADR Panel’s determinations will be binding and will set precedent for future disputes.
Although HRSA only has regulatory authority over 340B ceiling price calculations, manufacturer overcharge civil monetary penalties and ADR, they have issued guidance with respect to other 340B domains. Given its nonbinding nature, such guidance has been relatively weak and almost always met with litigation.[31] For example, in 2015 HRSA released their so-called “mega guidance” that addressed multiple areas of the 340B program including contract pharmacy compliance requirements, the eligible patient definition, and hospital eligibility criteria.[32] However, HRSA subsequently withdrew the guidance following a regulatory freeze issued by the Trump administration.[33] To resolve outstanding concerns related to patient definitions, DSH hospital eligibility, contract pharmacies, use of 340B funds, or other areas outside of HRSA’s authority, Congress can either expand HRSA’s regulatory authority or explicitly modify the 340B program through legislation.
Duplicate Discounts
For drugs prescribed to Medicaid patients, which includes fee-for-service (FFS) and managed Medicaid (MCO) plans, manufacturers are required to either provide rebates to states through the MDRP or sell them at a discounted price to covered entities through the 340B program, but not both. However, the challenges associated with accurate tracking and reporting of 340B discounts means that in some cases, a manufacturer will sell drugs to a covered entity at the 340B price and later pay a Medicaid rebate on the same drug; this is referred to as a “duplicate discount.”
Figure 6 provides a visual representation of a duplicate discount in 340B. In this case, the covered entity receives a discount on 340B drugs from the manufacturer and provides them to Medicaid patients in both FFS and MCO plans. If proper procedures were followed, the state Medicaid agency would not submit a claim to the manufacturer for these patients. However, in this case, the state Medicaid agency submits claims for the patients who already received 340B-discounted drugs and receives the Medicaid rebate from the manufacturer.
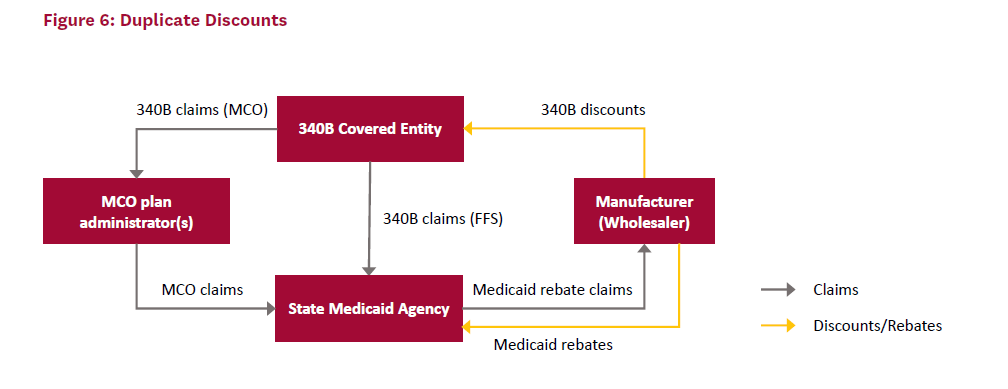
While duplicate discounts are prohibited, identifying and preventing them can be difficult due to lax record keeping and poor coordination between covered entities and state Medicaid agencies. Each state decides how its 340B covered entities handle Medicaid patients. If Medicaid patients are “carved in,” then covered entities can provide them with drugs purchased at 340B discounts, in which case the state should not claim MDRP rebates on them. If Medicaid patients are “carved out,” then the state can collect the MDRP rebates but covered entities cannot provide drugs purchased at 340B discounted prices to Medicaid patients. In the case of carve out, states receive the benefit of discounted drugs rather than covered entities.
Carve in/out rules vary across states, and specific policies vary within states: some states only carve out drugs dispensed by contract pharmacies but not covered entities; some carve in FFS Medicaid but carve out MCO plans; other states allow covered entities to choose which approach to use. For example, 45 states allow covered entities to decide whether to carve-in or carve-out FFS Medicaid, but only 25 states (out of 38 with MCO Medicaid) allow them to choose for MCO claims.[34] While the carve-out option can reduce the risk of duplicate discounts, 12 states do not require contract pharmacies to carve out FFS Medicaid drugs and 16 do not require them to carve out MCO drugs.[34]
When Medicaid drug benefits are carved in, covered entities and contract pharmacies are supposed to provide states with Medicaid drug utilization data so state agencies can exclude drugs purchased with 340B discounts from their manufacturer rebate requests. HRSA publishes the Medicaid Exclusion File (MEF) so states can identify which covered entities carve in Medicaid FFS and exclude their claims from rebate requests. However, the GAO and OIG both note that the MEF is not intended to be used to exclude 340B drugs provided to patients of MCO plans,[34, 35] which account for approximately 63% of Medicaid prescription drug spend,[36] and therefore cannot prevent all duplicate discounts. Moreover, 340B tracking in MCO plans can be difficult, particularly when prescriptions are filled through contract pharmacies. For example, 340B status is often unknown to the pharmacist at the point of sale, which prevents the use of 340B identifiers in claims or other reports.
States and contract pharmacies must then develop methods to identify 340B claims retroactively. The specific procedures used to identify 340B drugs in MCO plans vary by state, and in general use some combination of the MEF file, provider files developed by the state, and 340B claims identifiers.[34] The extent of duplicate discounts in the 340B program is unknown, but during the 1,536 audits conducted by HRSA between 2012 and 2019, there were 429 findings of noncompliance related to duplicate discounts.[10] Additionally, the potential for duplicate discounts increases as more covered entities and contract pharmacies participate in 340B. To address the problem of 340B duplicate discounts, GAO and OIG recommendations range from expanding HRSA audits to check for compliance with state Medicaid policies to requiring covered entities to work with manufacturers to repay MCO duplicate discounts. Because HRSA does not have regulatory authority over MCO plans and lacks resources to expand audits,[27] few changes have been made in the area of duplicate discount oversight. Others have proposed solutions aimed at reducing duplicate discounts such as establishing a national clearinghouse—which could be publicly or privately managed and funded—to identify 340B claims in MCO plans and remove them from Medicaid drug rebate claims.
Recent Growth in Contract Pharmacies and Their Role in 340B
Contract pharmacies provide a means for smaller covered entities without in-house pharmacies to access the 340B program and for covered entities with an in-house pharmacy to reach patients who opt to use external pharmacies. However, their growth since 2010 has been controversial in part because of HRSA guidance allowing for unlimited contract pharmacies. As of July 2017, approximately one-third of covered entities used a contract pharmacy and hospitals were more likely to have a contract pharmacy than other types of covered entities.[27] Covered entities are responsible for oversight of their contract pharmacies, including maintaining auditable records, conducting annual audits, and disclosing violations found during audits to HRSA.[37] While covered entities are required to register the names of their contract pharmacies with HRSA, the total number of contract pharmacy arrangements is unknown because covered entities are not required to submit contracts as part of the registration process.[27]
Although contract pharmacies increase the distribution of 340B discounted drugs, they also increase the complexity of identifying 340B prescriptions because they simultaneously serve patients of covered entities and non-340B providers. Consequently, contract pharmacies increase the risk of drug diversion, which occurs when 340B drugs are provided to a non-340B eligible patient. To prevent diversion, contract pharmacies must correctly identify which patients and prescriptions are 340B eligible; some covered entities use third party administrators (TPA) to help make these determinations. However, drug diversion still occurs: during audits conducted between 2012 and 2017, HRSA found that, out of 380 cases of drug diversion, 66% occurred at contract pharmacies.[27] Furthermore, 33% of these audits found insufficient contract pharmacy oversight by the covered entity. Although the GAO has recommended that HRSA provide additional requirements and guidance regarding contract pharmacy oversight, HRSA claims that it lacks regulatory authority over contract pharmacies and has not issued such guidance. Furthermore, HHS has deemed additional regulations too burdensome for covered entities.[27] As a result, additional oversight of contract pharmacies remains unlikely.
Beyond their role in drug diversion or duplicate discounts, contract pharmacies are also controversial because they were not originally meant to benefit financially from the 340B program. The share of major pharmacy chains such as Walgreens and CVS that serve as contract pharmacies has increased since 2010.[vi, 38] Specifically, Walgreens, CVS and Walmart accounted for 28%, 20% and 10% of contract pharmacy locations in 2020 (nearly 28,000 total).[39] Whether contract pharmacies pass 340B savings on to their low-income, uninsured or underinsured customers is unknown. Although comprehensive data are not available, in surveys of covered entities by the GAO and OIG,[27, 40 25] out of 55 and 8 out of 30, respectively, do not offer discounts to patients on 340B prescriptions filled at contract pharmacies.[vii]
Some have proposed limiting the number of contract pharmacies, particularly for hospitals. The total number of contract pharmacies could be based on geographical limits (e.g., distance from covered entity) or the size and insurance/income distribution of their patient population. To ensure low-income uninsured patients benefit from 340B, Congress could modify the legislation to require that covered entities develop charity care agreements with contract pharmacies and set their payments based on fair market values. Finally, additional visibility into the flow of 340B prescription funds and fee structures between covered entities, contract pharmacies/PBMs, and TPAs would improve oversight of the 340B program.
Concerns About DSH Hospitals Participating in 340B
Among all US hospitals that are potentially eligible for 340B, roughly 60% could qualify by demonstrating their DSH adjustment percentage–which is based in part on the share of low-income inpatient days–is above 11.75%.[41] DSH hospitals comprised a small share of covered entities prior to 2004, but their 340B participation rates have steadily increased over time.[42] In addition to comprising a growing share of covered entities, DSH hospitals account for roughly 80% of all 340B sales.[43] Yet despite large 340B sales growth since 2012, charity care provided by all US hospitals declined over the same period.[44] Unlike non-hospital covered entities, 340B DSH hospitals are not required to use 340B savings to serve vulnerable populations, nor are they required to report how 340B revenues are used.[7, 8] And while DSH requirements have remained constant, the composition of DSH hospitals participating in 340B has changed since 2004: DSH hospitals that participated in 340B prior to 2004 were larger, more likely to be public hospitals, and located in counties with lower income and higher levels of uninsured patients compared with DSH hospitals that began participating in 340B after 2004.[42, 45] These results suggest that hospitals that began participating in the 340B program after 2004 are more likely to serve wealthier and more insured populations, which is counter to the original intent of 340B savings being used to support care for vulnerable populations.
340B has affected other incentives for DSH hospitals. Medicare Part B spending per beneficiary is higher at 340B DSH hospitals compared with non-340B hospitals, which suggests DSH hospitals may shift prescribing behavior to improve profitability.[46] DSH hospitals can also expand their access to potential 340B patients by acquiring outpatient clinics, including oncology clinics. One study found that clinics affiliated with 340B DSH hospitals tend to serve communities with lower poverty rates and higher income levels than the 340B hospital.[45] Others present evidence that DSH hospitals near the 340B eligibility cutoff might be able to modify their patient mix to gain access to the 340B program.[47, 48] While none of these activities is explicitly disallowed, they are controversial because they are inconsistent with the original intent of the 340B program to benefit low-income populations.
Several solutions have been proposed to address concerns related to 340B DSH hospitals. In response to questions of whether new participants align with the program’s original intent, some have proposed a two-year moratorium on new hospital registrations or child sites.[49, 50] After the moratorium, hospital eligibility could be adjusted by limiting 340B eligibility to the hospitals with the highest DSH and capping the total number of participants. Others have suggested that DSH should no longer be used to determine hospital eligibility since it does not directly capture the degree to which hospitals serve low-income, uninsured or underinsured patients. In addition to modifying eligibility requirements, stricter reporting requirements would improve transparency into 340B programs. Suggested additional metrics that could be reported publicly include 340B revenues (net acquisition costs), 340B patient mix broken down by payer type, names and arrangements with contract pharmacies and third-party vendors, and annual reports on Medicare Part B claims subject to 340B.
Recent 340B Legislation and Other Developments
Over 50 pieces of federal legislation related to 340B have been introduced since 2011, but none has passed. Proposed legislation has focused on issues related to the scope of the 340B program, including updating the definition of patient eligibility, providing regulation for contract pharmacies or other third-party agreements, limiting the orphan drug exclusion,[51-53] requiring 340B drug claims modifiers, and updating reporting requirements, audits, and penalties for 340B violations.[49-53] The inability of Congress to pass legislation to address some of the issues with 340B coupled with the lack of systematic measures to improve 340B claims tracking and program integrity provides an opening for private sector solutions. While private sector solutions may help reduce duplicate discounts or drug diversion, they also introduce additional middlemen—who ultimately profit from 340B funds meant for covered entities—into an already complex 340B landscape.
In 2015, the Medicare Payment Advisory Committee (MedPAC) published a report that found the average minimum discount received by 340B hospitals equaled 22.5% of the average sales price (ASP).[8] In contrast, all hospitals—including 340B hospitals—receive reimbursement for Medicare Part B drugs equal to ASP plus 6%. To better align Medicare payments with the resource expenditures by hospitals, CMS issued a rule that would reduce reimbursement to 340B hospitals for Part B drugs to ASP minus 22.5% effective January 1, 2018.[54] The Medicare reimbursement cuts were deemed unlawful by a U.S. district court in 2019,[55] but the ruling was overturned in a 2020 appeal.[56] The Supreme Court has accepted a petition from the American Hospital Association to review the decision, and oral arguments related to the case are expected to be held in the fall of 2021.[57]
Lack of legislative change coupled with complexity and limited oversight of the 340B program has led several manufacturers to take steps limiting their exposure to diversion and duplicate discounts. In 2020, both Eli Lilly and AstraZeneca announced they would limit distribution of 340B-priced products to covered entities and their child sites and would no longer distribute 340B-priced products to contract pharmacies.[viii] Merck, Sanofi and Novartis have taken a different approach, notifying covered entities that they must provide contract pharmacy claims data to Second Sight Solutionsix to prevent duplicate discounts. Because these actions would essentially have manufacturers scaling back the 340B program, they were met with complaints from hospital and provider organizations such as the American Hospital Association and 340B Health, and 28 state Attorneys General.[58, 59] In response, HHS issued advisory guidance stating that manufacturers participating in 340B must provide 340B discounts to covered entities’ contract pharmacies.[4] Eli Lilly, Sanofi and AstraZeneca have all separately challenged the HHS guidance in court.[60]
Conclusion
Although the drug discounts provided by 340B have provided many covered entities with funds to support their operations, whether these funds are used in a manner consistent with the intent of the 340B program remains an area of controversy. Vaguely worded legislation coupled with HRSA’s limited regulatory authority have created a program that leaves implementation open for interpretation and provides limited meaningful oversight activities. Over the last 30 years, the growth of the 340B program coupled with a lack of legislative reforms has created a challenging situation for private stakeholders and left the terms guiding the transfer of billions of dollars in value annually up to private actors and the courts. Until Congress can pass legislation that effectively modifies the 340B program or its oversight, manufacturers and covered entities, particularly hospitals, will likely continue to test the bounds of the program. There are valid concerns with the 340B program related to transparency, diversion, and duplicate discounts, particularly through contract pharmacies and hospitals. However, the 340B Program has also provided significant resources to many providers and allowed them to better serve millions of patients. Congressional reform efforts will need to take both perspectives into account to sustainably modify the program.
Footnotes
- [i] Under this program, manufacturers who want their drugs covered under Medicaid must enter into a rebate agreement with the Secretary of the Department of Health and Human Services (HHS). The implication of tying 340B participation with MDRP is that manufacturers would only be able to access the Medicaid market if they participate in 340B.
- [ii] This requires that the offsite clinic is owned by the hospital. Outpatient facilities that file their own Medicare Cost Report under a separate provider number are not eligible for 340B even if they are affiliated with the 340B hospital.
- [iii] This implies the covered entity maintains the health records of the patient.
- [iv] DSH hospitals are exempt from this requirement.
- [v] Generic drug ceiling prices are calculated in a similar manner, but use 13% rather than 23.1% in the formula.
- [vi] Contract pharmacy contracts are made at the pharmacy level; contracts made with a chain pharmacy will be for specific locations, but not all locations of the entire chain.
- [vii] In contrast, 17 of 23 covered entities surveyed by the GAO with an in-house pharmacy reported offering discounts at their in-house pharmacy, including 4 who do not offer discounts at their contract pharmacy.
- [viii] Both companies provide a carve out allowing covered entities without in-house pharmacies to designate a single contract pharmacy partner for 340B distribution.
- [ix] Second Sight Solutions is a prescription drug information technology company that collects 340B contract pharmacy claims on behalf of manufacturers. It was founded by Aaron Vandervelde, who has written articles and conducted work for 340B manufacturer and oncology advocacy groups.
References
- Health Resources & Services Administration. 340B drug pricing program. 2021 [cited 2021 Jun 24]; Available from: https://www.hrsa.gov/opa/index.html.
- Fein, A.J. Exclusive: the 340B program soared to $38 billion in 2020—up 27% vs. 2019. 2021 [cited 2021
Jun 24]; Available from: https://www.drugchannels.net/2021/06/exclusive-340b-program-soared-to-38.html. - Health Resources & Services Administration. 340B program hospital registration instructions. 2019 [cited
2021 May 3]; Available from: https://www.hrsa.gov/sites/default/files/hrsa/opa/hospital-registration-instructiondetails.
pdf. - Department of Health & Human Services. Advisory opinion 20-06 on contract pharmacies under the 340B program. 2020 [cited 2021 May 3]; Available from: https://www.hhs.gov/guidance/sites/default/files/hhsguidance-
documents/340B-AO-FINAL-12-30-2020_0.pdf. - Health Resources & Services Administration. Notice regarding 340B drug pricing program—contract pharmacy services. Federal Register. 75(43). Mar 5, 2010 [cited 2021 May 5]; Available from: https://www.govinfo.gov/content/ pkg/FR-2010-03-05/pdf/2010-4755.pdf.
- United States Government Accountability Office. Drug pricing: manufacturer discounts in the 340B program offer benefits, but federal oversight needs improvement. 2011 [cited 2021 May 3]; Available from: https://www.gao.gov/assets/gao-11-836.pdf.
- Community Access National Network. 340B Commission’s final report on the 340B drug discount program: the issues spurring discussion, stakeholder stances and possible resolutions. 2019 [cited 2021 May 24]; Available from: https://docs.google.com/gview?url=http://www.tiicann.org/pdf-docs/2019_CANN_340B_Commission_Final-Report-v5_03-07-19.pdf&embedded=true.
- Medicare Payment Advisory Commission. Report to the Congress: overview of the 340B drug pricing program. 2015; Available from: http://www.medpac.gov/docs/default-source/reports/may-2015-report-to-the-congressoverview-
of-the-340b-drug-pricing-program.pdf?sfvrsn=0. - Health Resources & Services Administration. Notice regarding section 602 of the Veterans Health Care Act of 1992 patient and entity eligibility. Federal Register. 61(207). Oct 4, 1996 [cited 2021 May 3]; Available from: https://www.govinfo.gov/content/pkg/FR-1996-10-24/pdf/96-27344.pdf.
- United States Government Accountability Office. Drug pricing program: HHS uses multiple mechanisms to help ensure compliance with 340B requirements. 2020 [cited 2021 Aug 23]; Available from: https://www.gao.gov/assets/gao-21-107.pdf.
- HR 34 – 21st Century Cures Act, P.L. 114-255, §3022, 114th Congress. 2016.
- Environmental Protection Agency. Pesticides; certification of pesticide applicators. Federal Register. 82(2). Jan 4, 2017
[cited 2021 May 3]; Available from: https://www.govinfo.gov/content/pkg/FR-2017-01-04/pdf/2016-30332.pdf. - 340B Prime Vendor Program. The PVP supports the 340B drug pricing program. [cited 2021 Aug 23]; Available from: https://www.340bpvp.com/about-340b-and-pvp.
- Fein, A.J. Exclusive: the 340B program hits $16.2 billion in 2016; now 5% of U.S. drug market. 2017 [cited 2021 Jun 24]; Available from: https://www.drugchannels.net/2017/05/exclusive-340b-programhits-162-billion.html.
- National Association of Community Health Centers. What matters for health centers: 340B drug program. 2018 [cited 2021 May 3]; Available from: https://www.nachc. org/focus-areas/policy-matters/340b.
- Malouin, R.A., et al., Impact of the 340B pharmacyprogram on services and supports for persons served by hemophilia treatment centers in the United States. Maternal and Child Health Journal, 2018. 22(9): p. 1240-1246.
- Ryan White Clinics for 340B Access. Resources for covered entities in response to recent actions taken by drug manufacturers. [cited 2021 May 3]; Available from: https://www.rwc340b.org/.
- 340B Health. 2020 340B health annual survey: 340B hospitals use savings to provide services and improve outcomes for low-income and rural patients. [cited 2021 May 3]; Available from: https://www.340bhealth.org/files/340B_Health_Survey_Report_2020_FINAL.pdf.
- Clark, B., et al., Medication adherence among HIV/AIDS patients receiving 340B-purchased antiretroviral medications. Value in Health, 2016. 19(3): p. A8.
- Rana, I., von Oehsen, W., Nabulsi, N. A., Sharp, L. K., Donnelly, A. J., Shah, S. D., Stubbings, J., Durley, S. F. A comparison of medication access services at 340B and non-340B hospitals,. 2021 [cited 2021 Aug 23]; Available from: https://reader.elsevier.com/reader/sd/pii/S1551741121001169?token=C4C8AB45AB44202E1070A36B6AD46F29EF81A3F8762A7999A3F52EE43600FC2D983156538EC80E94680099126F9504F9&originRegion=us-east-1&originCreation=20210823170621.
- Conti, R.M., Nikpay, S. S., Buntin, M. B., Revenues and profits From Medicare patients in hospitals participating in the 340B drug discount program, 2013-2016. JAMA Network Open, 2019. 2(10): p. e1914141-e1914141.
- Statement of Adam J. Fein. National Commission on 340B, The Community Access National Network. 2018 [cited 2021 May 3]; Available from: http://www.tiicann.org/pdf-docs/2018_CANN_340B_Commission_Fein-Testimony-13-June-2018.pdf.
- Patient Protection and Affordable Care Act, P. L. 111–148,111th Congress. 2010.
- Church, R.P., Ruskin, A. D., Richardson, L. D., Hamscho, V. K. 340B update: HRSA indicates it lacks authority to enforce340B program guidance. 2020 [cited 2021 Aug
23]; Available from: https://www.natlawreview.com/article/340b-update-hrsa-indicates-it-lacks-authority-toenforce-340b-program-guidance. - Office of Inspector General. Deficiencies in the oversight of the 340B drug pricing program. 2005 [cited 2021 May 3]; Available from: https://www.hrsa.gov/sites/default/files/opa/programrequirements/reports/oversightdeficiencies102005.pdf.
- Centers for Medicare & Medicaid Services. Modernizing Part D and Medicare Advantage to lower drug prices and reduce out-of-pocket expenses. Federal Register. 83(231). Nov 30, 2018 [cited 2021 May 3]; Available from: https://www.govinfo.gov/content/pkg/FR-2018-11-30/pdf/2018-25945.pdf.
- United States Government Accountability Office. Drug discount program: federal oversight of compliance at 340B contract pharmacies needs improvement. 2018 [cited 2021 May 3]; Available from: https://www.gao.gov/assets/gao-18-480.pdf.
- Church, R.P., Hamscho, V. K., Richardson, L. D., Ruskin, A. D. 340B update: federal court halts 340B administrative dispute resolution rule. 2021 [cited 2021 May 3]; Available from: https://www.natlawreview.com/article/340b-updatefederal-court-halts-340badministrative-dispute-resolution-rule.
- Pharmaceutical Research and Manufacturers of America vs. Cochran at al. Maryland District Court, 2021 [cited2021 May 3]; Available from: https://phrma.org/-/ media/Project/PhRMA/PhRMA-Org/PhRMA-Org/PDF/D-“F/Docket-1-12221-PhRMA-v-Cochran-etal–Complaint.pdf.
- Environmental Protection Agency. Hazardous and solid waste management system: disposal of CCR; a holistic approach to closure Part B: alternate demonstration for unlined surface impoundments; correction. Federal Register. 85(240). Dec 14, 2020 [cited 2021 May 3]; Available from: https://www.govinfo.gov/content/pkg/FR-2020-12-14/pdf/2020-25810.pdf.
- Breuer, J.R. 340B orphan drug rule invalid. 2014 [cited 2021 May 3]; Available from: https://www.natlawreview.com/article/340b-orphan-drug-rule-invalid.
- Department of Health & Human Services. 340B drug pricing program omnibus guidance. Federal Register. 80(167). Aug 28, 2015 [cited 2021 Aug 23]; Available from: https://www.govinfo.gov/content/pkg/FR-2015-08-28/pdf/2015-21246.pdf.
- Ellison, A. Trump administration withdraws 340B megaguidance: 6 things to know. 2017 [cited 2021 Aug 23]; Available from: https://www.beckershospitalreview.com/finance/trump-administration-withdraws-340b-megaguidance-6-things-to-know.html.
- United States Government Accountability Office. 340B drug discount program: oversight of the intersection with the Medicaid drug rebate program needs improvement. 2020 [cited 2021 May 3]; Available from: https://www. gao.gov/assets/710/706831.pdf.
- Department of Health & Human Services. State efforts to exclude 340B drugs from Medicaid managed care rebates. 2016 [cited 2021 Jun 25]; Available from: https://oig.hhs.gov/oei/reports/oei-05-14-00430.pdf.
- Medicaid and CHIP Payment and Access Commission. MACStats: Medicaid and CHIP data book. 2020 [cited 2021 May 3]; Available from: https://www.macpac.gov/macstats/.
- Health Resources & Services Administration. Contract pharmacy services. 2018 [cited 2021 Jun 24]; Available from: https://www.hrsa.gov/opa/implementation/contract/index.html.
- Fein, AJ. How hospitals and PBMs profit—and patients lose—from 340B contract pharmacies. 2020 [cited 2021 May 3]; Available from: https://www.drugchannels.net/2020/07/how-hospitals-and-pbms-profitand.html.
- Fein, AJ. Walgreens and CVS top the 28,000 pharmacies profiting from the 340B program. Will the unregulated party end? 2020 [cited 2021 May 3]; Available from: https://www.drugchannels.net/2020/07/walgreens-andcvs- top-28000-pharmacies.html.
- Office of Inspector General. Contract pharmacy arrangements in the 340B program. 2014 [cited 2021 May
3]; Available from: https://oig.hhs.gov/oei/reports/oei-05-13-00431.pdf. - United States Government Accountability Office. Drug discount program: characteristics of hospitals participating
and not participating in the 340B program. 2018 [cite 2021 Sep 15]; Available from: https://www.gao.gov/products/gao-18-521r. - Nikpay, S., M. Buntin, and R.M. Conti, Diversity of participants in the 340B drug pricing program for US hospitals. JAMA Internal Medicine, 2018. 178(8): p. 1124-1127.
- Alliance for Integrity and Reform of 340B. Benefiting hospitals, not patients: an analysis of charity care provided by hospitals enrolled in the 340B discount program. 2016 [cited 2021 May 3]; Available from: https://340breform.org/userfiles/May%202016%20AIR340B%20Avalere%20Charity%20Care%20Study.pdf.
- Fein, A.J. New HRSA data: 340B program reached $29.9 billion in 2019; now over 8% of drug sales. 2020[cited 2021 May 3]; Available from: https://www.drugchannels.net/2020/06/new-hrsa-data-340bprogram-reached-299.html.
- Conti, R.M. and P.B. Bach, The 340B drug discount program: hospitals generate profits by expanding to reach more affluent communities. Health Affairs, 2014. 33(10): p. 1786-1792.
- United States Government Accountability Office. Medicare Part B drugs: action needed to reduce financial incentives to prescribe 340B drugs at participating hospitals. 2015 [cited 2021 May 5]; Available from: https://www.gao.gov/assets/gao-15-442.pdf.
- Nikpay, S.S., et al. A prescription for manipulation? Impact of the 340B drug discount program on hospitals. in 7th Annual Conference of the American Society of Health Economists. 2018.
- Mulligan, K., J.A. Romley, and R. Myerson, Access to the 340B drug pricing program: is there evidence of strategic hospital behavior? BMC Research Notes, 2021. 14(228): p. 1-5.
- Helping Ensure Low-income Patients have Access to Care and Treatment Act, in S.2312, 115th Congress. 2017-2018.
- 340B Protecting Access for the Underserved and Safety-net Entities Act, in H.R.4710, 115th Congress. 2017-2018.
- Closing Loopholes for Orphan Drugs Act, in .R.853, 117th Congress. 2021-2022.
- 340B Protection and Accountability Act of 2019, in H.R.1559, 116th Congress. 2019-2020.
- Safe Routes Act of 2019, in H.R.2453, 116th Congress. 2019-2020.
- Environmental Protection Agency. Procedures for Chemical Risk Evaluation Under the Amended Toxic Substances Control Act. Federal Register. 82(138). Jul 20, 2017 [cited 2021 Aug 23]; Available from: https://www.govinfo.gov/content/pkg/FR-2017-07-20/pdf/2017-14337.pdf.
- United States District Court for the District of Columbia, American Hospital Association, et al., v. Alex M. Azar II, United States Secretary of Health and Human Services, et al., in No. 20-5193. 2020.
- Cook, E.J., Schnelle, S. J., Ren, C. DC circuit upholds opps reimbursement reductions for 340B drugs. 2020 [cited 2021
Aug 23]; Available from: https://www.mwe.com/insights/dc-circuit-upholds-opps-reimbursement-reductions-for-340b-drugs/. - Ruskin, A.D., Church, R. P., Richardson, L. D., Hamscho, V. K. 340B update: Supreme Court accepts certiorari in 340B payment reduction case. 2021 [cited 2021 Aug 23]; Available from: https://www.natlawreview.com/article/340b-update-supreme-court-accepts-certiorari-340b-payment-reduction-case.
- American Hospital Association. AHA, others file lawsuit over drug companies’ refusing 340B discounts. 2020 [cited 2021 May 3]; Available from: https://www.aha.org/news/headline/2020-12-14-aha-others-file-lawsuit-over-drugcompanies- refusing-340b-discounts.
- Attorney General Letter to U.S. Department of Health and Human Services. Drug manufacturers’ actions violating 340B program pricing program requirements. 2020 [cited 2021 May 3]; Available from: https://oag.ca.gov/sites/default/files/340B%20Multistate%20Letter%2012.14.2020_FINAL%5B1%5D.pdf.
- Sullivan, T. Drug manufacturers sue HHS over 340B advisory opinion. Policy & Medicine 2021 [cited 2021 May 3]; Available from: https://www.policymed. com/2021/02/drug-manufacturers-sue-hhs-over-340badvisory-opinion.html.

You must be logged in to post a comment.