Key Takeaways
- Economic and cultural factors limit access to cancer screening and erode trust in the healthcare system among minority patients.
- Multi-cancer early-detection blood-based tests may mitigate disparities in early cancer diagnosis experienced across minority communities if offered in collaboration with regular screening schedules to detect aggressive tumors that afflict minority patients at higher rates than the general population.
- MCED tests may also mitigate disparities by eliminating the need for access to specialists and ensuring consistent quality and accuracy of test results, thereby reducing discrepancies in patient care found across other types of screening.
- If test adoption is lower among physicians in minority communities, or if insurance coverage is low, then multi-cancer early-detection blood-based testing could disproportionately benefit white patients and increase, rather than reduce, racial disparities in early cancer detection.
Abstract
Despite universal recommendations from the U.S. Preventive Services Task Force for routine screening for breast, cervical and colon cancers, racial and ethnic minorities are less likely to receive these screenings and more likely to be diagnosed with cancer at later stages than their white counterparts. Given that one of the most important prognostic factors for cancer remains the stage of disease at initial diagnosis, screening disparities may be contributing to disparities in cancer mortality suffered by Black and Hispanic Americans.
The advent of multi-cancer early-detection blood-based tests, which can detect the presence of previously unscreened cancers, may help mitigate some of these disparities. In this paper, we summarize current screening systems and existing disparities in early detection. We then explore whether access to multi-cancer early-detection blood-based tests could expand access to screening and reduce racial and ethnic disparities.
Introduction
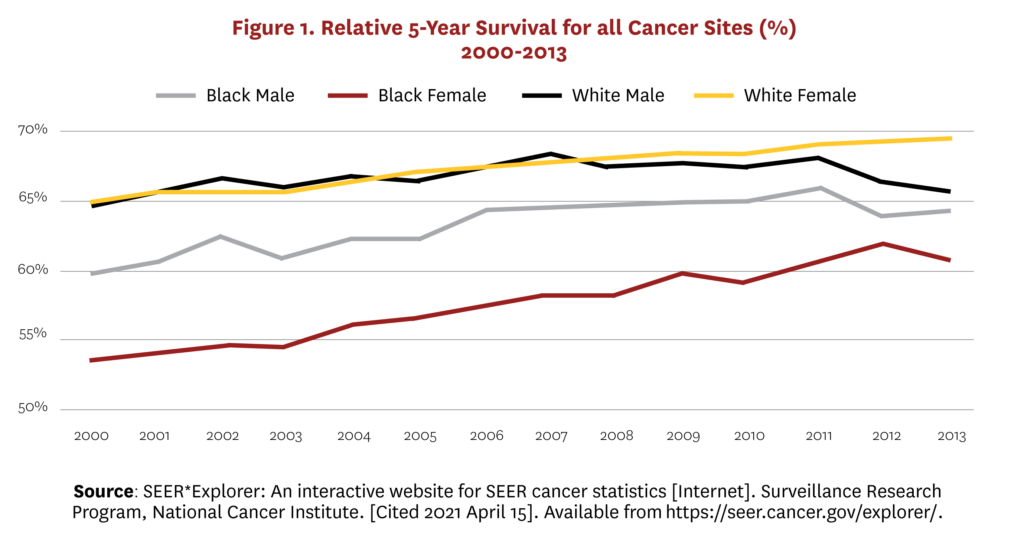
Racial and ethnic disparities in cancer mortality and diagnosis represent significant problems for the U.S. healthcare system. Although reductions in smoking have narrowed racial disparities in cancer mortality over the last few decades, the cancer death rate remains 19% and 13% higher in non-Hispanic (NH) Black men and women, respectively, compared to their NH white counterparts.[1] Figure 1 illustrates similar disparities in five-year survival rates for Black male and female cancer patients relative to white patients. This is due, at least in part, to the disparity in early cancer detection rates between Blacks and whites.[2]
Despite notable advances in cancer therapies, one of the most important prognostic factors remains the stage of detection.[3] For example, patients with breast or colon cancer who are diagnosed while the disease is still localized have a five-year survival rate similar to the general population. But when the disease is diagnosed in advanced stages, the five-year relative survival rate falls to only 30%.[4, 5]
The U.S. Preventive Services Task Force (USPSTF) recommends routine screening for breast, cervical and colon cancers for the entire population and for lung, prostate and ovarian cancer in patients with significant risk factors. Not surprisingly, we see mortality rates falling faster in these specific cancers than in those without routine screening. However, the benefits from current screening technologies have not accrued equally across all patients. Racial and ethnic minorities are less likely to receive recommended cancer screenings and more likely to be diagnosed with cancer at later stages than their white counterparts.[6, 7] The consequences of lower screening rates within racial/ethnic minority communities are especially severe because Blacks and Hispanics experience a higher incidence of cervical cancer, colon cancers and aggressive triple-negative forms of breast cancer than whites, making early detection especially important.[8, 9] In this paper, we ask whether technical advances in early cancer detection have the potential to improve screening in minority communities and increase early diagnosis.
Economic and cultural factors that limit access to screening and erode trust in the healthcare system among minority patients contribute to disparities in early cancer detection within minority communities.[10-12] Not only are Black and Hispanic patients less likely than white patients to have health insurance and thus financial support for the recommended screenings, but those who are screened are more likely to be treated in lower-quality and understaffed facilities compared to white patients.[13-15] The care they receive is lower quality, the screenings are less reliable, and follow-up tests are frequently delayed or never scheduled.[13] Finally, religious and cultural beliefs within some minority communities can discourage preventive care and promote distrust in the healthcare system.[16] This makes it less common for patients to advocate for the referrals necessary to comply with USPSTF screening recommendations. For example, fatalistic beliefs about cancer and embarrassment around screening procedures are commonly found in the Hispanic community.[17, 18] Furthermore, research shows that Spanish-speaking patients with low English fluency are less likely to have up-to-date cancer screenings.[19]
New opportunities for enhanced early cancer detection may be on the horizon in the form of blood-based tests, which can detect tumors in previously unscreened cancers and broaden access to screening within currently screened cancers. These new multi-cancer early-detection (MCED), blood-based tests rely on advances in the analysis of cell-free DNA (cfDNA) and machine learning to detect and locate a number of cancer types from a simple blood sample.[23] Several tests are under development that vary in their detection methodology, cancers detected and performance metrics, but overall the new tests offer very high specificity (i.e., very few false positives) and a range of sensitivities (true cancers detected, 16% to 100%) depending on the tumor type and stage.[23] If approved, MCED blood-based tests could be used in addition to current recommended screening approaches to identify cancers that would have otherwise been missed.[24]
Blood-based tests and other laboratory services are not plagued by the same level of racial disparities as other medical services, such as cancer screenings, and thus have the potential to reduce some of the disparities in early cancer detection. In fact, the rate of errors in lab testing in general has dropped dramatically in the last 40 years with the automation of labs.[20] And although some differences in test results are reported across labs based on calibration of equipment, the literature does not suggest that this results in racial inequities.[21] Furthermore, most hospitals and independent laboratories are unlikely to run specialized and advanced tests such as those for blood-based MCED. As a result, samples will have to be shipped from the physician’s office to a centralized lab for processing. Although the shipping takes time, the use of centralized labs ensures the same quality of testing for all patients. Errors can still occur in the initial blood draw before shipping, but studies suggest these errors are more common with inpatient care where the blood may be drawn by someone without phlebotomy training compared to outpatient care settings such as primary care visits.[22] These factors suggest that blood-based tests may provide access to early cancer screening without the need for referral to a specialist and without concern over unequal access to high-quality testing/results.
In what follows, we summarize the current screening recommendations and technologies for cancers with routine screening. We also examine the current rates of early detection among screened and non-screened tumors as reported in the published literature. We rely on each source’s racial and ethnic groupings, which are not consistent across sources. Thus, some reported statistics are not separated for Hispanics, Blacks and whites. Finally, we explore current barriers to cancer screening and whether access to new MCED blood-based tests have the potential to increase screening and reduce racial and ethnic disparities among these cancers.
The Current State of Routine Screening Recommendations
Many physicians’ groups, patient advocacy groups and other medical organizations have created early cancer screening recommendations, but the USPSTF’s recommendations have the greatest influence on insurance coverage and thus patient access. The USPSTF is an independent, volunteer panel of experts overseen by the federal Health and Human Services’ Agency for Healthcare Research and Quality (AHRQ) that provides evidence-based recommendations about clinical preventive services such as screenings, counseling services and preventive medications. Although the Centers for Medicare & Medicaid Services was previously empowered to add preventive services to Medicare and Medicaid, it was the Patient Protection and Affordable Care Act (ACA) that formalized coverage requirements for most private and government insurance plans. For example, with few exceptions all private and public insurance plans are required to cover preventive services with a grade of A or B from the USPSTF without any cost sharing for patients. This means that most health plans cover USPSTF’s broad recommendations for breast, colon and cervical cancer screening at no cost. However, the data show that uptake of screening has been slow and differs by tumor type and by patient racial identity.
For example, breast cancer is the most common type of cancer in the U.S. and is one of the leading causes of cancer death.[25] These facts motivate the USPSTF’s recommendation for biennial mammography breast cancer screening for all women ages 50–74. However, the benefits of regular screening and early detection have not been experienced equally by all women. Despite the fact that NH white women have a slightly higher incidence of breast cancer than NH Black women and both groups have similar self-reported mammogram screening rates (around 65% of women are up to date on screening), NH Black women are about 15% less likely to have their cancers detected in localized stages of disease compared to NH white women, and have a 41% higher mortality rate than NH white women.1 This difference in death rates is the result of a number of factors, including a later stage of diagnosis, higher prevalence of obesity and other comorbid conditions, and less access to high-quality cancer care among NH Black women.[26–28]
Similarly, colorectal cancer (CRC) is the second-leading cause of cancer death in the U.S., and African Americans have a greater incidence of the disease and a higher mortality rate compared to white Americans.[29] In fact, the CRC mortality rate has declined dramatically (39%) for whites over the last 60 years but has steadily increased for the Black population.10 The mortality reductions among whites are due in part to the uptake of regular CRC screening recommendations for all Americans over age 50.[30, 31] The screening can be done using a variety of methods with varying levels of patient burden. The less invasive options look for blood and DNA abnormalities in stool samples and can often be completed in the patient’s home. Colonoscopies require complete and unpleasant cleansing of the colon in the days before the test and sedation during the exam, but offer the most accurate results.[32] Despite broad CRC screening recommendations and advances in screening technologies, Blacks are more likely be diagnosed with late-stage disease than whites.[33-35] The literature suggests this disparity in early diagnosis persists because screening rates are lowest among racial and ethnic minorities.[10, 36] The racial disparity in compliance with screening recommendations is exacerbated by the fact that Black and Hispanic Americans, on average, are diagnosed at a younger age with a more aggressive form of the disease than whites.
Cervical cancer is often highlighted as a success story for cancer-screening programs. The mortality rate from cervical cancer has declined steadily over the last 40 years due to widespread use of cytological screening and curative treatments for precancerous lesions.[37] However, the success of cervical screening has not been shared equally by all women. Studies have shown lower rates of screening for older women and minority women.11 Hispanic women have lower rates of cytological screening than Black women, and both minority groups are more likely to be diagnosed with later-stage disease than white women.[37]
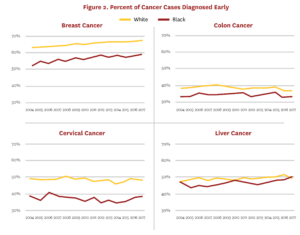
The USPSTF also recommends screening for lung cancer in adults with an extensive smoking history; however, to date, the remaining tumor types are generally unscreened. In fact, about half of cancer diagnoses and deaths in the U.S. come from cancers that do not have a recommended screening test,[38] and disparities in mortality rates within these cancers are less likely to result from differences in early detection. Figure 2 shows early detection rates of Blacks in the U.S. relative to whites for breast, cervical and colon cancers, which have early screening recommendations, and liver cancer, which does not. Access policies for breast, cervical and colon cancer screening appear to have created disparities that we do not see in liver cancer. In other words, the introduction of screening exams for breast, colon and cervical cancers reduced mortality rates, but also introduced racial disparities because policies around screening adoption, costs and insurance coverage created unequal access to the screening technologies.
Barriers to Screening Minorities
As these early-detection statistics show, minority patients are not fully benefiting from the USPSTF screening recommendations and their cancers in some cases are slipping through the cracks. These patients are not receiving the quantity and quality of screening required to achieve consistent early detection of their cancers. One of the most commonly cited barriers to early diagnosis with cancer screening among Blacks and Hispanics is the quality of care they receive. This is particularly true for women seeking breast cancer screening. On average, white women are more likely than Black or Hispanic women to have mammograms at academic facilities, facilities that rely exclusively on breast-imaging specialists to read mammograms and facilities where digital mammography is available.[13] The facilities also vary considerably in their ability to follow up on abnormal screens and refer patients for additional testing. One study found that these differences in screening facilities accounted for most of the discrepancies in late-stage diagnoses between Black and white women, after controlling for neighborhood.[14] Additionally, lower-quality mammogram facilities also take longer, on average, to follow up on abnormal test results.[39]
Another likely cause of disparities in early cancer diagnosis between white and Black patients is the higher rate of more aggressive tumor types among minority patients. For example, in breast cancer there is a higher incidence of triple-negative tumors (TNT) among Black women compared to white women.[9] TNTs tend to be more aggressive and impact a younger population,8 suggesting that they may progress too quickly to be detected early under the current screening technologies and recommendations. The current guidelines from the USPSTF are for biennial breast cancer screening in all women ages 50–74. These guidelines were set after reviewing the benefits and risks of mammogram technology and balancing the potential of earlier diagnosis with the high potential for false-positive test results. However, TNTs progress to late stages faster than other tumors, and the two-year gap may be too long for early diagnosis in these more aggressive tumors.[40]
Similarly, among colon cancer patients, Black and Hispanic Americans, on average, are diagnosed at a younger age with a more aggressive form of the disease than whites.[41] In fact, researchers have found specific genes that are more likely to be mutated in colon cancers from Black patients than whites and are associated with a higher chance of metastatic disease and relapses.[42]
Another significant barrier to cancer screening among minority patients is general distrust in the healthcare system and a lack of awareness about cancer screening, causes and early signs/symptoms.[11, 43, 44] This is particularly true for colon and cervical cancer, where minority patients frequently hold misperceptions about the relationship between sexual activity and cancer risk, which generates a stigma around screening and testing.[45] Research also suggests that religious and cultural beliefs within some minority communities can discourage preventive care and promote general distrust in the healthcare system.[16]
Finally, financial barriers may also prevent minority patients from receiving adequate cancer screening.[46] By law, Medicare must cover all preventive services with a recommendation from the USPSTF with a grade A or B, such as the cancer screenings we have discussed.[49] State Medicaid programs also have incentivizes to cover the same A-rated and B-rated services at no cost to patients in order to receive additional funds from the federal government.[50] Additionally, the ACA requires employer-based private insurance policies to cover preventive services at no cost to patients.[51] Thus, the absence of any health insurance represents the largest financial barrier to cancer screening, and studies show that Hispanic and Black patients are more likely than other patients to be without insurance.[46-48] Although many national, state and local programs are designed to promote cancer screening in low-income and uninsured communities, they are only able to reach a small portion of those patients lacking care.[46]
Potential Benefits of MCED Blood-Based Tests
In principle, MCED blood-based tests can address several of the current challenges in cancer screening within minority communities. For example, the tests do not require specialist visits and primary care physicians can use MCED blood-based tests to start a conversation with patients about cancer risks and determine when and how often to test. Specifically, physicians can assess each patient’s risk for cancer based on age, family history and comorbidities, and choose to use the blood test between currently recommended cancer screenings for those patients at highest risk for cancer, particularly in its most aggressive forms. Currently, the false-positive rate of mammograms makes it too risky for physicians to recommend more frequent usage among patients, but MCED blood-based tests feature high specificity and little burden on the patient in terms of additional appointments. Colonoscopies require extensive and uncomfortable colon cleansing and are expensive; it may not be realistic to recommend them more frequently, but regular MCED blood-based tests could be effective in detecting aggressive tumors between procedures.
MCED blood-based tests can also be used in younger patients at high cancer risk before other types of screening are recommended. The tests are not tumor-specific, so one blood test could screen younger patients for a number of different cancers with little risk or discomfort. The false-positive rates (specificity) are so low for these tests that the risk of wasteful follow-up testing is limited.
The quality of the lab-based blood test is also more likely to be uniform regardless of the physician office where the test is administered, thereby avoiding the discrepancies in patient care found across other types of screening centers. This will also be important in evaluating the potential of MCED blood-based tests to address the tremendous unmet need in unscreened cancers. The quality and consistency of results may be easier to achieve, but equity in early diagnosis can only be achieved if all patients have broad access to the tests. In fact, if patients do not have equitable access, these tests could mainly benefit advantaged communities and thereby exacerbate existing disparities in early detection and create new inequalities in previously unscreened tumors.
Conclusion
Economic and cultural factors limit access to cancer screening and erode trust in the healthcare system among minority patients. As a result, minority patients are less likely to have their screen-available cancers diagnosed at early stages where treatments are most successful. The goals of health policy should be to eliminate these barriers and improve trust in screening in minority communities. However, these cultural and systematic changes may be achieved only slowly over time even in a best-case scenario. In the meantime, MCED blood-based tests may offer hope to overcome some of the disparities because they can be a flexible tool for physicians in the battle to reduce or eliminate barriers to screening within minority communities. For example, MCED blood-based testing can be offered through primary care physicians without the need for a referral or separate visit to a specialist. In rural communities, access to specialists can be limited, increasing the benefits of convenient blood-based tests for minority patients. All blood samples would be sent to a central lab for processing, ensuring consistent quality and accuracy of the results regardless of where the sample was drawn.
The new tests could be offered between current recommended screenings to reduce the risks caused by more aggressive tumors that afflict minority communities at higher rates than the general population. The tests are not tumor-specific and could therefore offer broad cancer screening between recommended screenings. For example, women at high risk for breast cancer could have MCED blood-based tests between biennial mammograms. MCED blood-based tests could also be used to start screening minority populations for cancer, including colon cancer, at a younger age. Provided equitable access to MCED blood-based tests, they could complement existing screening regimens recommended by the USPSTF to better address patients’ individual cancer risks.
Despite the potential of MCED blood-based tests to increase cancer screening and reduce racial disparities, realization of its promise is far from certain. It will depend on whether access is provided broadly to minority patients and whether physicians use the tests equally for all patients at high risk of cancer. If test adoption is lower among physicians in minority communities, or if insurance coverage is lower among minority patients, then MCED blood-based testing could increase racial disparities in early cancer detection rather than reducing them. As MCED blood-based tests are introduced, more research will be needed to track their use and understand their real-world impact on barriers to cancer screening.
Support for this work was provided by the USC Schaeffer Center for Health Policy & Economics. The views expressed herein are those of the authors, and do not represent the views of the funders. The Schaeffer Center is supported by a wide variety of public and private entities and donors, including companies developing blood-based tests for cancer. Lakdawalla reports personal fees or research support from Amgen, Biogen, Genentech, GRAIL, Edwards Lifesciences, Novartis, Otsuka, Perrigo, and Pfizer and holds equity in Precision Medicine Group, which provides consulting services to firms in the life sciences.
This paper has undergone the Schaeffer Center white paper quality assurance process, led by Emmett Keeler, Schaeffer Center Senior Fellow and Quality Assurance Director. In addition to his review, the paper was reviewed by two scholars not affiliated with the Schaeffer Center.
References
- DeSantis, C.E., et al., Cancer statistics for African Americans, 2019. CA Cancer J Clin, 2019. 69(3): p. 211-233.
- Ward, E., et al., Cancer disparities by race/ethnicity and socioeconomic status. CA: a cancer journal for clinicians, 2004. 54(2): p. 78-93.
- Crosby, D., et al., A roadmap for the early detection and diagnosis of cancer. The Lancet Oncology, 2020. 21(11): p. 1397-1399.
- The American Cancer Society medical and editorial content team. Lung Cancer Survival Rates. Lung Cancer 2021 January 29, 2021 [cited 2021 March 9, 2021]; Available from: https://www.cancer.org/cancer/lung-cancer/detection-diagnosis-staging/survival-rates.html.
- The American Cancer Society medical and editorial content team. Survival Rates for Breast Cancer. 2021 January 27, 2021 [cited 2021 March 9, 2021]; Available from: https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html.
- Fiscella, K., et al., Disparities in preventive procedures: comparisons of self-report and Medicare claims data. BMC Health Services Research, 2006. 6(1): p. 1-8.
- Siegel, R.L., K.D. Miller, and A. Jemal, Cancer statistics, 2019. CA: a cancer journal for clinicians, 2019. 69(1): p. 7-34.
- Dietze, E.C., et al., Triple-negative breast cancer in African-American women: disparities versus biology. Nature Reviews Cancer, 2015. 15(4): p. 248-254.
- Lund, M.J., et al., Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res and Treat, 2009. 113(2): p. 357-370.
- Tammana, V.S. and A.O. Laiyemo, Colorectal cancer disparities: issues, controversies and solutions. World journal of gastroenterology: WJG, 2014. 20(4): p. 869.
- Bastani, R., et al., Increasing cervical cancer screening among underserved women in a large urban county health system: can it be done? What does it take? Medical care, 2002: p. 891-907.
- Doescher MP, Saver BG, Franks P, Fiscella K. Racial and ethnic disparities in perceptions of physician style and trust. Arch Fam Med. 2000 Nov-Dec;9(10):1156-63. doi: 10.1001/archfami.9.10.1156. PMID: 11115223.
- Rauscher, G.H., et al., Disparities in screening mammography services by race/ethnicity and health insurance. Journal of women’s health, 2012. 21(2): p. 154-160.
- Warnecke, R.B., et al., Multilevel examination of health disparity: the role of policy implementation in neighborhood context, in patient resources, and in healthcare facilities on later stage of breast cancer diagnosis. Cancer Epidemiology and Prevention Biomarkers, 2019. 28(1): p. 59-66.
- Molina, Y., A. Silva, and G.H. Rauscher, Racial/ethnic disparities in time to a breast cancer diagnosis: the mediating effects of healthcare facility factors. Medical care, 2015. 53(10): p. 872.
- Shelton, R.C., et al., The influence of sociocultural factors on colonoscopy and FOBT screening adherence among low-income Hispanics. Journal of health care for the poor and underserved, 2011. 22(3): p. 925.
- Allen, J.D., et al., Religious beliefs and cancer screening behaviors among Catholic Latinos: Implications for faith-based interventions. Journal of health care for the poor and underserved, 2014. 25(2): p. 503.
- Gorin, S.S., Correlates of colorectal cancer screening compliance among urban Hispanics. Journal of behavioral medicine, 2005. 28(2): p. 125-137.
- Fiscella, K., et al., Disparities in health care by race, ethnicity, and language among the insured: findings from a national sample. Medical care, 2002: p. 52-59.
- International Organization for Standardization/Technical Specification, Medical Laboratories-Reduction of Error Through Risk Management and Continual Improvement. ISO/TS 22367: 2008, ISO Geneva, Switzerland.
- Power, P., Do Blood Test Results Differ When Processed at Different Labs?, in Ask the expert. 2016.
- Plebani, M., Errors in clinical laboratories or errors in laboratory medicine? Clinical Chemistry and Laboratory Medicine (CCLM), 2006. 44(6): p. 750-759.
- Liu, M.C., Transforming the landscape of early cancer detection using blood tests—Commentary on current methodologies and future prospects. British Journal of Cancer, 2021.
- Chen, X., et al., Non-invasive early detection of cancer four years before conventional diagnosis using a blood test. Nature Communications, 2020. 11(1): p. 3475.
- American Cancer Society, Cancer facts & figures 2019. . 2019, American Cancer Society: Atlanta, GA.
- Sengupta, R. and K. Honey, AACR Cancer Disparities Progress Report 2020: Achieving the Bold Vision of Health Equity for Racial and Ethnic Minorities and Other Underserved Populations. Cancer Epidemiology Biomarkers & Prevention, 2020. 29(10): p. 1843-1843.
- Tammemagi, C.M., et al., Comorbidity and survival disparities among black and white patients with breast cancer. JAMA, 2005. 294(14): p. 1765-1772.
- Shavers, V.L. and M.L. Brown, Racial and ethnic disparities in the receipt of cancer treatment. Journal of the National Cancer Institute, 2002. 94(5): p. 334-357.
- Centers for Disease Control and Prevention, An Update on Cancer Deaths in the United States, US Department of Health and Human Services: Atlanta, GA.
- Zauber, A.G., The impact of screening on colorectal cancer mortality and incidence: has it really made a difference? Digestive Diseases and Sciences, 2015. 60(3): p. 681-691.
- Doubeni, C.A., et al., Effectiveness of screening colonoscopy in reducing the risk of death from right and left colon cancer: a large community-based study. Gut, 2018. 67(2): p. 291-298.
- Mayo Clinic Staff, Colon cancer screening: Weighing the options, in Health Information. 2020, Mayo Clinic.
- Doubeni, C.A., et al., Racial differences in tumor stage and survival for colorectal cancer in an insured population. Cancer: Interdisciplinary International Journal of the American Cancer Society, 2007. 109(3): p. 612-620.
- Govindarajan, R., et al., Racial differences in the outcome of patients with colorectal carcinoma. Cancer, 2003. 97(2): p. 493-498.
- McAlearney, A.S., et al., Racial differences in colorectal cancer screening practices and knowledge within a low‐income population. Cancer: Interdisciplinary International Journal of the American Cancer Society, 2008. 112(2): p. 391-398.
- Benarroch‐Gampel, J., et al., Colonoscopist and primary care physician supply and disparities in colorectal cancer screening. Health Services Research, 2012. 47(3pt1): p. 1137-1157.
- Musselwhite, L.W., et al., Racial/ethnic disparities in cervical cancer screening and outcomes. Acta Cytologica, 2016. 60(6): p. 518-526.
- Siegal, R., K.D. Miller, and A. Jemal, Cancer statistics, 2012. Ca Cancer J Clin, 2014. 64(1): p. 9-29.
- Rauscher, G.H., et al., Beyond the mammography quality standards act: measuring the quality of breast cancer screening programs. American Journal of Roentgenology, 2014. 202(1): p. 145-151.
- Miglioretti, D., et al., Risk of less-favorable breast tumor characteristics with biennial versus annual mammography. JAMA Oncology, 2015. 1(8): p. 1069-1077.
- Goldman, R.E., J.A. Diaz, and I. Kim, Perspectives of colorectal cancer risk and screening among Dominicans and Puerto Ricans: stigma and misperceptions. Qualitative Health Research, 2009. 19(11): p. 1559-1568.
- Wang, Z., et al., Adverse clinical outcome associated with mutations that typify African American Colorectal Cancers. JNCI: Journal of the National Cancer Institute, 2016. 108(12): p. djw164.
- Lee, H.Y., et al., Racial Disparities in Cervical Cancer Screening: Implications for Relieving Cervical Cancer Burden in Asian American Pacific Islander Women. Cancer nursing, 2019. 42(6): p. 458-467.
- Luque, J.S., et al., Cultural beliefs and understandings of cervical cancer among Mexican immigrant women in Southeast Georgia. Journal of Immigrant and Minority health, 2015. 17(3): p. 713-721.
- Jones, R.M., et al., Patient-reported barriers to colorectal cancer screening: a mixed-methods analysis. American journal of Preventive medicine, 2010. 38(5): p. 508-516.
- Fiscella, K., et al., Eliminating disparities in cancer screening and follow-up of abnormal results: what will it take? Journal of Health Care for the Poor and Underserved, 2011. 22(1): p. 83-100.
- Mead, H., et al., Racial and ethnic disparities in US health care: A chartbook. New York, NY, The Commonwealth Fund, 2008.
- Robinson, J.M. and V. Shavers, The role of health insurance coverage in cancer screening utilization. Journal of Health Care for the Poor and Underserved, 2008. 19(3): p. 842-856.
- Jensen, Gail A., et al. “A slow start: use of preventive services among seniors following the Affordable Care Act’s enhancement of Medicare benefits in the US.” Preventive medicine 76 (2015): p. 37-42.
- Gates, A., U. Ranji, and L. Snyder. “Coverage of preventive services for adults in Medicaid.” Kaiser Family Foundation (2014).
- Kaiser Family Foundation. “Preventive services covered by private health plans under the Affordable Care Act.” (2015).

You must be logged in to post a comment.