Key Takeaways
- Alzheimer’s disease (AD) clinical trials are more complicated, costly, and slower than trials for other diseases.
- The AD patient journey spans three ecosystems: community, healthcare, and clinical trial.
- Across these ecosystems, key barriers include limited patient awareness, lack of clear diagnosis, and infrequent trial referrals which hinder trial success.
- Surmounting these barriers would increase the number and diversity of patients who have access to clinical trials, support the successful completion of more trials, and accelerate the approval of innovative therapies.
- Stakeholders in the Alzheimer’s research community must take a holistic view of these barriers and collaborate to eliminate them.
A blog about this white paper is available here and a press release is available here.
Abstract
Randomized clinical trials are a cornerstone of the drug-approval process, but in Alzheimer’s disease (AD) they present a particular challenge. Alzheimer’s trials tend to be slower to enroll participants, take longer to complete, and are more expensive than trials in most other therapeutic categories. In fact, approximately 99% of eligible patients are never referred to or consider participating in an AD clinical trial. To be effective, new approaches to AD clinical trials must surmount barriers to participation and bring as many people as possible into, and through, the AD clinical-trial ecosystem. The Alzheimer’s research community—including patient organizations, healthcare providers, researchers, government, and innovators—must collaborate to make this possible.
Overview
Alzheimer’s disease (AD) is an epidemic that is rapidly overwhelming our older population, their caregivers and families, and the healthcare system. Approved treatments for AD can modestly reduce symptoms in the later stages of dementia and are palliative in nature, but do not slow or reverse the progression of disease. Despite the need for better pharmacological interventions and major efforts across academia and industry, no new drugs for the disease have entered the global market in more than 15 years.
Without clinical trials, the approval of new therapies in any area is impossible—and Alzheimer’s trials are especially challenging. As will be discussed in this paper, they are slower to enroll participants, take longer to complete, and are more expensive than trials in most other therapeutic areas—issues that are exacerbated by the barriers that prevent subjects from participating in AD clinical trials in the first place.1
Accordingly, we set out to understand the patient journey to and through an AD clinical trial to identify the barriers to successful trials and understand how we might overcome them. The data presented in this analysis come from several clinical trial databases, interviews with over 60 stakeholders, as well as quantitative survey results from nearly 900 respondents (see Appendix A for more details).
This paper examines why AD clinical trials are particularly challenging. It focuses on the patients and subjects themselves, and the barriers that arise as a potential trial subject navigates their way into and through an AD clinical trial.
We find the main barriers to more efficient AD clinical trials are those which keep patients from ever reaching the trials. While this issue is not particular to AD, this paper will discuss how the unique and complex nature of AD—such as increased fear and stigma of the disease and uncertain diagnoses— exacerbates these issues. The barriers identified fall into three categories: those that stigmatize AD and/ or limit patient awareness of the disease, hamper clear diagnoses, or curb referrals to AD clinical trials. Better understanding these barriers will help to identify new approaches to AD clinical trials that can bring as many people as possible into, and through, the AD clinical-trial ecosystem.
What is AD and How Is It Diagnosed?
Doctors and researchers classify AD into stages based on the clinical presentation. In the first stage, known as “preclinical AD,” biomarkers identify precursors to clinical manifestation, such as amyloid-beta plaques in patients’ brains.2 Patients in this preclinical stage experience no symptoms, and it can last for 10–15 years.3
The next stage, which can last for five years or more, is known as “mild cognitive impairment” (MCI) due to AD or “prodromal AD.” In this stage, affected patients show mild cognitive symptoms characteristic of MCI, and if combined with a positive pathophysiological marker of AD can be defined as “prodromal AD.”4 Often, people mistakenly attribute these mild symptoms to “normal” aging, which can delay diagnosis and treatment.
In the final stage of the disease, known as “clinical AD,” progressive impairments in memory and behavior can impair AD patients’ ability to function independently. Clinical AD can last for 5–15 years, until the patient dies from complications related to AD or another cause.5
For patients with symptoms of AD such as impaired memory or cognition, healthcare providers usually reach a diagnosis via a process of elimination: they assess a patient’s symptoms, perform neurocognitive screening or testing, perform laboratory tests to rule out other conditions that can affect cognitive function, and interview friends and family to get a sense of changing abilities and behaviors over time.6
AD results from the progressive degeneration of brain cells, which can be observed in magnetic resonance imaging (MRI) and computerized tomography (CT) scans, and eventually leads to the symptoms associated with the disease, detectable via cognitive assessments and neuropsychological evaluations. However, these tests are not definitive. In fact, clinicians used to say that AD could only be definitively diagnosed in an autopsy after a patient’s death, allowing doctors to say whether a patient’s symptoms were due to AD or another type of dementia. Today, positron emission tomography (PET), scans use radioactive tracers to detect biomarkers like amyloid-beta plaques in vivo in the brain; researchers can also detect amyloid-beta in preclinical AD patients’ cerebrospinal fluid (CSF) and plasma.7 Both approaches now allow doctors and researchers to identify the tell-tale pathological changes associated with AD in the brain while subjects are still alive, and perhaps before any symptoms appear.
Trends in AD Clinical Trials
Compared to clinical trials in other therapeutic areas, AD trials are complicated, expensive, and slow.1,8
Screening procedures including neurocognitive tests, MRIs, PET scans, and CSF tests are expensive and time-consuming, especially for asymptomatic patients, and they screen out many more patients than they screen in. The screen-failure rate of a clinical trial refers to the percent of subjects who undergo screening but do not meet the enrollment criteria of a trial, and is a key driver of costs for clinical trials across disease areas. Mild AD trials have an average screen-failure rate of 44%, and preclinical trials have an average screen-failure rate of 88%. This is in part driven by the stringent screening criteria of many AD trials, such as amyloid-beta PET positivity and specific cutoffs for neurocognitive status, which cannot be easily identified before presenting to a trial.1 This results in significant work for the site in order to recruit even one eligible subject to a trial.
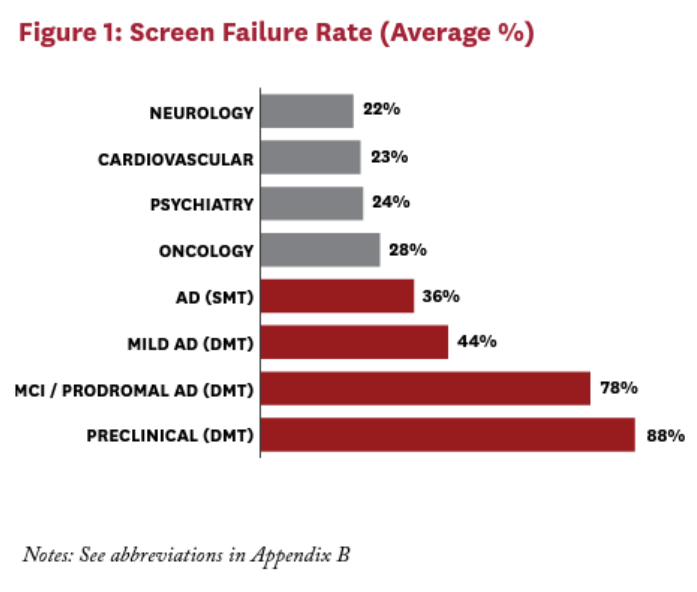
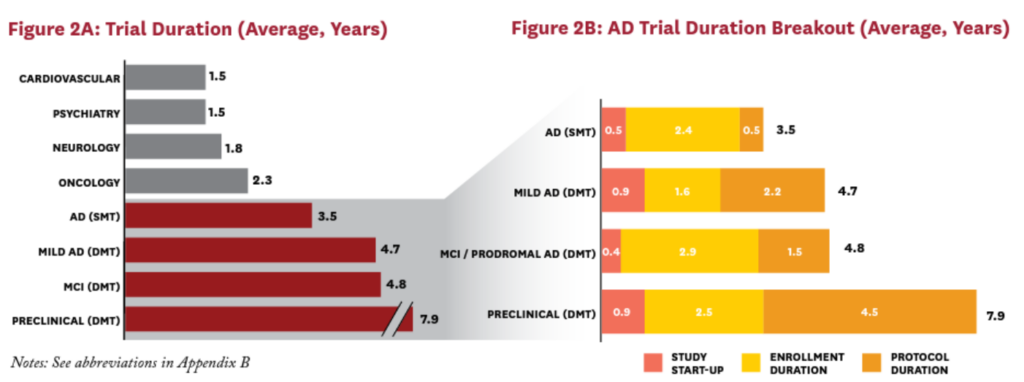
These high screen-failure rates are one reason why AD disease-modifying therapy (DMT) trials tend to last significantly longer than trials in other therapeutic areas (Figure 1). Screening procedures also take time. Usually timeslots for the imaging modalities must be booked in advance, and waiting times for a scan can be as long as 12 weeks. Another factor is the variable rate of progression of the disease: researchers trying to demonstrate that their treatment can slow AD’s progression even further may require years—not months—to do so9 (Figure 2).
AD trials cost more per patient than trials in many other therapeutic areas. Patient-screening costs account for 50–70% of total per-patient costs for AD trials. For instance: each cognitive test can cost trial sponsors $600; each MRI can cost sponsors $2,400; and each amyloid-pathology test can cost anywhere from $1,250 (for a CSF test) to $8,000 (for a PET scan). (These costs are what the study sponsor typically pays the investigator per procedure.) Screen failures at each step mean more tests for more patients, and more costly trials for sponsors.1
Most large AD drug trials are multicenter clinical trials, and therefore the performance of individual trial sites contributes to the overall efficiency and costs of a given trial. In general, site performance varies greatly and is trial-specific: a top-performing site in one trial could perform very poorly for another. A top-performing clinical trial site has the personnel and time to dedicate to the work of clinical trials, as well as the PET and other infrastructure the research requires. It also displays high levels of investigator motivation and sponsor engagement, which can vary from one trial at a given site to the next. Most of all, a top-performing site boasts strong relationships between site staff and patients—a critical element of patient retention in AD trials. However, in all cases individual sites can only impact the efficiency of a trial once a patient has entered the clinical trial system, and upstream barriers preventing the awareness, diagnosis and referral of patients along the journey to a clinical trial are critical to unlocking the potential of any single site.
The Patient Journey to AD Trial: Three Ecosytems
Before we can identify, understand, and surmount the obstacles to advance Alzheimer’s research, we must understand the current AD patient journey from when symptoms appear (or even before) to when a diagnosis is made and then to completion of a clinical trial. We organize the AD patient journey into three “ecosystems”: the community ecosystem, the healthcare ecosystem, and the clinical-trial ecosystem (Figure 3).
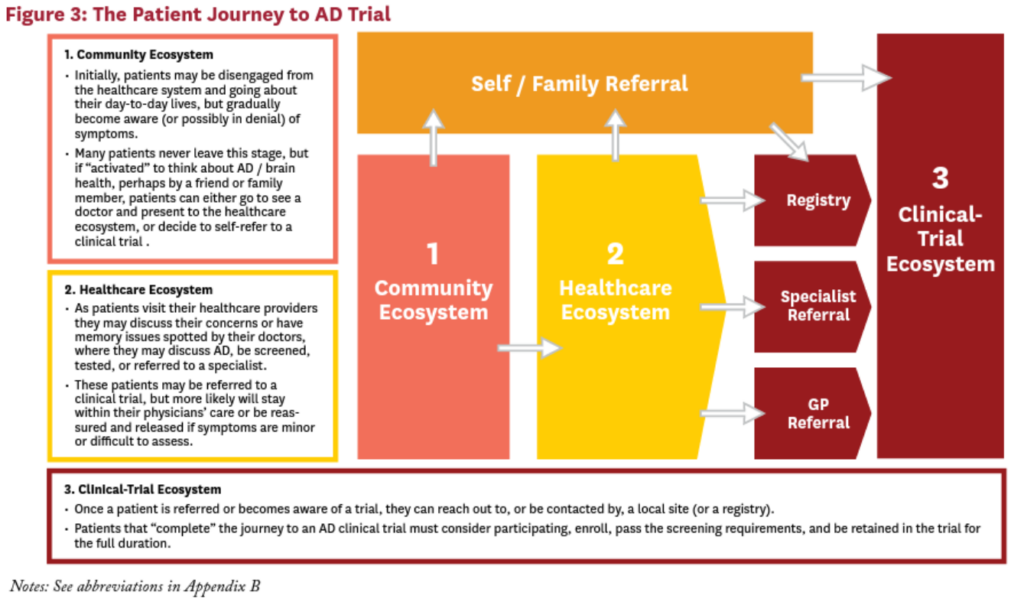
Community Ecosystems
In their day-to-day lives, some patients (or their family members or informal caregivers) may gradually become aware that they are experiencing mild or moderate cognitive impairment or other symptoms that could be associated with AD. Many patients never leave this ecosystem—especially if they do not yet have symptoms or are in denial about their symptoms. However, if something triggers them to think about AD and brain health, they may visit a doctor or, in rarer cases, self-refer to a registry or clinical trial.
Within the community ecosystem, the subjective nature of potential symptoms during the earliest stages of the disease presents challenges on both ends of the spectrum: some potential subjects may be in denial, while others may be the “worried well.” The latter group refers to subjects who are worried they have dementia, but in fact are neurologically and neuropsychologically normal, constituting a good percentage of screen failures in trial-selection procedures.10
Healthcare Ecosytem
Patients who visit their healthcare providers might raise their concerns about the symptoms of cognitive decline they are experiencing or concerned about. Alternatively, doctors might spot cognitive impairments in patients who either have not noticed or are ignoring their symptoms, but are visiting their healthcare providers for another reason. In either case, patients in the healthcare ecosystem may start a conversation about AD and be screened, tested, or referred to a specialist. These patients may be referred to a clinical trial, but only if the treating physician is the principal investigator (PI) of a trial, is aware of the trials ongoing in the surrounding area, or they know the local PIs who are involved in running these trials. In most cases, however—especially if symptoms are minor or difficult to assess—patients typically remain in the care of their doctor.
Clinical-Trial Ecosystem
Once a patient becomes aware of an AD clinical trial in their area, either on her own or via a doctor’s referral, she can enroll. Patients who enroll, pass the screening requirements, and start and comply with treatment and monitoring for the full trial duration are said to “complete” the journey through an AD clinical trial. As within the community and healthcare ecosystems, patients can be lost at multiple steps along this process—whether due to the time commitment and subject burden, perceived risks, stringent screening criteria or simply due to misinformation and/or a lack of information regarding clinical trials in general.
Seven Key Barrier to AD Clinical Trials
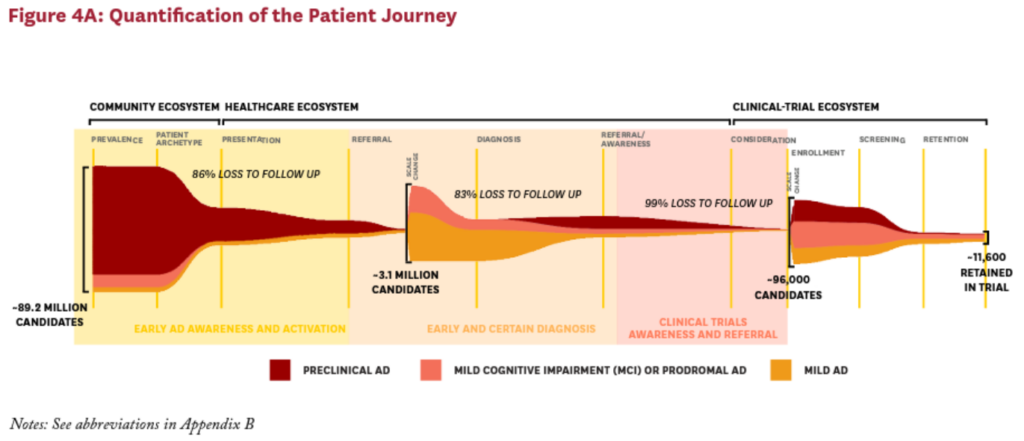
Across these three ecosystems, we identified seven key barriers that can hinder patients and researchers alike, limiting the flow of subjects to clinical trials. The insights of patients, subjects and their caregivers, clinical trial personnel and physicians were used to quantitatively model the patient journey to an AD clinical trial and understand where along the patient journey potential trial subjects are lost. Within each ecosystem, these key barriers cause approximately 99% of eligible subjects to be lost before ever enrolling in a clinical trial (Figure 4).
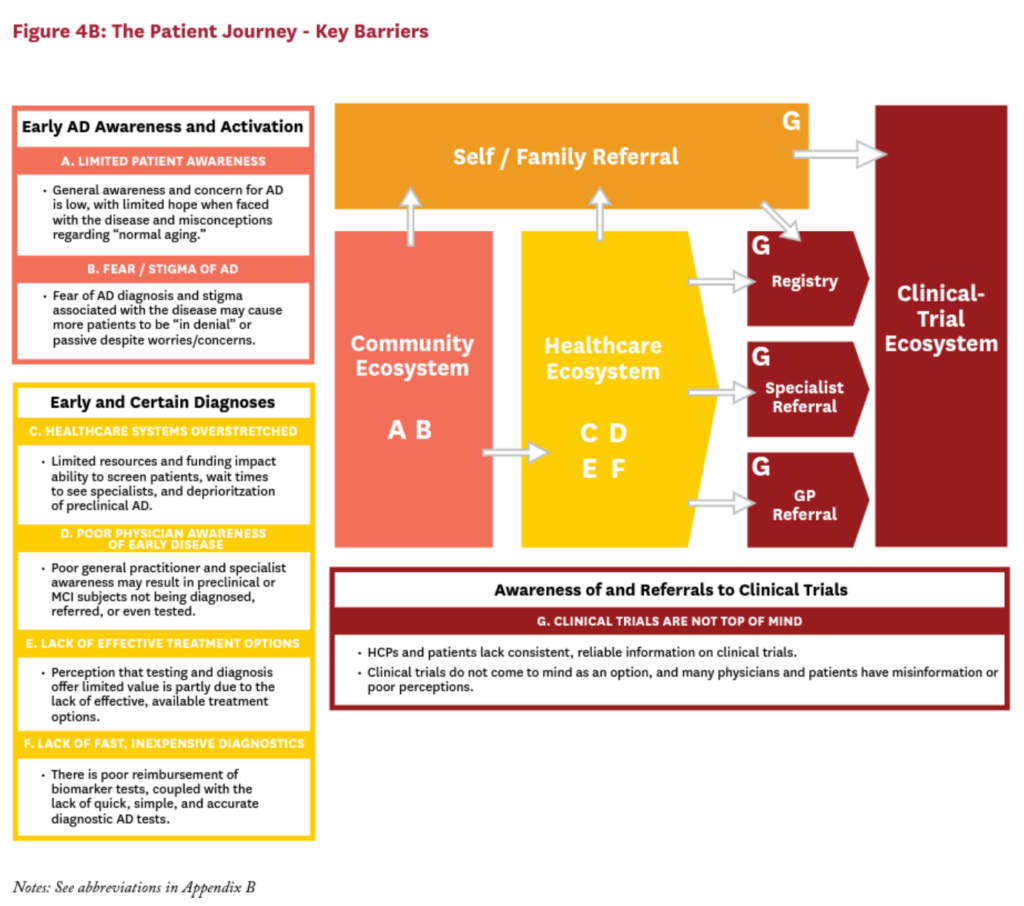
Community Ecosystem
In the community ecosystem, there are two main barriers to AD clinical trial participation:
- A. Limited Awareness of Early AD: People with preclinical AD experience no symptoms. They may progress to the next stage of the disease, in which they experience mild symptoms—yet most individuals surveyed believe that memory loss and other cognitive impairments are a consequence of “normal aging.”11 In both cases, many patients do not realize they may have AD; as a result, they do not contact their doctor or find their way to a clinical trial until their symptoms get much worse, sometimes years or even decades later.
- B. Fear of an AD Diagnosis: More than 60% of MCI patients and more than 50% of patients with mild AD said they had downplayed or ignored their memory issues before their diagnosis.11 In the absence of effective treatments, the thought of an AD diagnosis is terrifying. The stigma associated with the disease has real consequences for patients’ everyday lives: they fear losing their jobs, their health-insurance coverage, their driver’s licenses (and their day-to-day independence); and their ability to make their own decisions about medical care and finances.
Consequently, potential subjects often remain in the community ecosystem, with no motivation to seek diagnosis, care, or treatment for AD. At the very beginning of the patient journey, these patients are lost as potential trial subjects. In fact, of the 89.2 million possible AD clinical trial subjects eligible for a preclinical, prodromal or mild AD clinical trial in the U.S., only 12.5 million ever leave the community ecosystem and enter the healthcare ecosystem due to concerns about their brain health, memory decline or potential symptoms of AD.12
Healthcare Ecosystem
Of those patients who make it to the healthcare ecosystem, they encounter four major barriers to AD clinical trial participation:
- C. Healthcare Systems Overstretched: Nearly 70% of healthcare providers report that they do not always have the time or the resources to discuss AD with their patients—especially with patients who show no visible symptoms of declining cognitive health.11 As one general physician put it: “I don’t have time to start worrying about people who are basically well.” Also—at least in the U.S., where specialists may perform patient screening for AD— the incentive structure of the healthcare system can discourage a general practioner (GP) from referring a patient to a specialist for screening. If referring a patient to a neurologist or a clinical trial means losing that patient, then every referral equates to potential revenue loss for a general physician.
- D. Poor Physician Awareness of Early Disease: Many physicians do not currently identify potential AD patients until they are already experiencing mild or moderate symptoms of the disease. In fact, most physicians, including both GPs and specialists, are unfamiliar with or do not use the term “preclinical AD” in their practice. Although the term “preclinical AD” is currently intended for research use, limited awareness of this category of subjects may be limiting the likelihood that physicians will investigate or discuss possible AD unless overt symptoms are present.11 Boosting physician awareness that key biological changes can be underway well before a patient shows symptoms may be critical to early identification of the disease—and to funneling patients to preclinical trials from the community ecosystem.
- E. Lack of Effective Treatment Options: Without an effective treatment for AD, many patients and their physicians fail to see the benefits in screening for the disease.11 In fact, 75% of the physicians surveyed pointed to the dearth of good treatment options to explain why they do not often screen for AD. As one U.S. neurologist said: “Even if [patients] have a clear, confident diagnosis, there is no treatment—then so what?” The value of timely diagnosis has been well-reported in the literature, and the perceived lack of treatments on the part of physicians may be contributing to delayed or under-diagnosis of AD.13-15 Because of this perception, many patients never reach the trials that might bring us closer to better treatments and therapeutics.
- F. Lack of Fast, Inexpensive Diagnostics: Confirming an AD diagnosis is a long, complicated and expensive process. Patients rarely get the biomarker tests, either via CSF tests or via PET scanning, that can definitively diagnose AD pathology. Instead, most patients are diagnosed using a process of elimination, relying heavily on observable symptoms associated with later stages of the disease. Typically, a physician will review a patient’s medical history; conduct cognitive and neuropsychiatric tests (which can be time-consuming, error-prone, and subjective); and conduct MRIs or CT scans to rule out other conditions.
Only 2–3% of preclinical AD patients and 10–12% of patients in the MCI stage undergo biomarker testing (PET scans or lumbar-puncture CSF tests), in part because of restricted access to those tests, their high costs, and the lack of treatments for diagnosed patients.11 (In the U.S., some 65% of patients who undergo amyloid-imaging tests pay for them out of pocket.16) Without a quick, simple, accurate test that payors will reimburse, many patients and physicians will not bother to confirm their AD diagnosis.
Together, these four barriers cause some 83% of the potential subjects who made it to the healthcare ecosystem to be lost before they can be funneled into the clinical-trial ecosystem.12
Before patients reach the clinical-trial ecosystem, they face one final but critical barrier:
- G. Awareness of and Referrals to Clinical Trials: Many healthcare providers lack consistent, reliable information on clinical trials. As a result, clinical trials are not often top-of-mind as a treatment option. Most physicians report awareness of ongoing AD clinical trials generally; however, only 5–10% report awareness of ongoing AD clinical trials in their area, and even fewer report awareness of where and how to refer patients into those trials. As one UK physician said: “I will never and have never in 30 years thought about referring a patient to clinical trial.”11
Even if patients are aware of or referred to a trial, they may not be inclined to join it. In fact, approximately 80% of patients have never considered participating in a trial.11 Even if they meet the rigorous patient-screening requirements, patients can shy away from the perceived risks or side effects of the therapies being tested—and if they are not informed about clinical trials in general, they may not think the benefit to future patients outweighs the possible risks in the present.
This specific barrier is not unique to AD, but trials in other therapeutic areas could possibly provide important insights to inform how best to address it.
Conclusions
As a result of all these barriers, just a small subset of motivated, engaged, informed, and qualified patients are likely to join a trial. As a consequence, the subjects who end up participating in AD clinical trials tend to be those with more positive perceptions of clinical trials, and may be more educated and/or engaged with respect to their brain health, with potential implications for the generalizability of clinical trial findings.11 In all, the system misses approximately 99% of all eligible patients somewhere along the journey to clinical trial.12
There are several limitations of this research that warrant noting. First, this research is largely based on primary research from a small sample of respondents that may not be representative of the broader population. In addition, the current scope of work focuses heavily on the U.S., and therefore generalizing findings to other countries may not be appropriate. In addition, the current work focuses on the barriers and challenges along the patient journey to a clinical trial, without careful consideration of the potential solutions to address these challenges. This topic warrants more significant discussion than can be adequately covered in this paper, including evaluating, designing, implementing, and monitoring the success of potential solutions. This will be the focus of a forthcoming paper. Nonetheless, the current work emphasizes critical issues currently limiting potential subjects’ ability to navigate the journey to an AD clinical trial.
To be effective, new approaches to AD clinical trials must surmount the barriers to participation and bring as many people as possible into, and through, the AD clinical-trial ecosystem. That means every member of the Alzheimer’s research community—including patient organizations, healthcare providers, researchers, government, and innovators—must take a holistic view of the process from start to finish, with close attention to the challenges faced by patients and their caregivers prior to ever considering participation in a clinical trial. Then, these stakeholders must work together to reform it: increasing awareness of AD, especially early AD, among the public; enabling healthcare providers to provide early and certain AD diagnoses; and/ or facilitating patient awareness of, referral to, and participation in clinical trials. When they do, they will bring us closer to a successfully commercialized DMT in AD.
References
- IQVIA, IQVIA Infosario Database. June 2018.
- Dubois, B., et al., Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimer’s & Dementia, 2016. 12(3): p. 292-323.
- Sperling, R., E. Mormino, and K. Johnson, The evolution of preclinical Alzheimer’s disease: implications for prevention trials. Neuron, 2014. 84(3): p. 608-622.
- Albert, M.S., et al., The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging‐Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 2011. 7(3): p. 270-279.
- National Institute on Aging. What are the signs of Alzheimer’s disease? 2017 [cited 2020 July]; Available from: https://www.nia.nih.gov/health/what-are-signs-alzheimers-disease.
- Mayo Clinic. Diagnosing Alzheimer’s: How Alzheimer’s is diagnosed. 2019 [cited 2020 July]; Available from: https://www.mayoclinic.org/diseases-conditions/alzheimers-disease/in-depth/alzheimers/art-20048075.
- Alzheimer’s Association. Earlier diagnosis. 2020 [cited 2020 July]; Available from: https://www.alz.org/ alzheimers-dementia/research_progress/earlier-diagnosis.
- Citeline. Citeline Global Clinical Trials Database. [cited 2018 January]; Available from: https://pharmaintelligence.informa.com/products-and-services/data-and-analysis/citeline.
- Cummings, J., H. Gould, and K. Zhong, Advances in designs for Alzheimer’s disease clinical trials. American Journal of Neurodegenerative Disease, 2012. 1(3): p. 205.
- Ahmed, S., et al., Memory complaints in mild cognitive impairment, worried well, and semantic dementia patients. Alzheimer Disease & Associated Disorders, 2008. 22(3): p. 227-235.
- IQVIA, Alzheimer’s Disease Patient and Physician Survey. June 2018.
- IQVIA, IQVIA Alzheimer’s Patient Journey and Clinical Trial Model. July 2018.
- Dubois, B., et al., Timely diagnosis for Alzheimer’s disease: a literature review on benefits and challenges. Journal of Alzheimer’s Disease, 2016. 49(3): p. 617-631.
- Petersen, R.C., et al., Mild cognitive impairment: clinical characterization and outcome. Archives of Neurology, 1999. 56(3): p. 303-308.
- Mueller, S.G., et al., Ways toward an early diagnosis in Alzheimer’s disease: the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Alzheimer’s & Dementia, 2005. 1(1): p. 55-66.
- Decision Resources Group, Mechanisms by which patients gain access to amyloid imaging according to surveyed neurologists. August 2016.
- U.S. National Library of Medicine. ClinicalTrials.gov [cited 2018 January]; Available from: https://www. clinicaltrials.gov/.
Appendix A: Summary of Key Information Sources & Methodology
Three key databases provided clinical-trial metrics and data. IQVIA provided detailed metrics from IQVIA-run clinical trials. IQVIA (NYSE:IQV) is a leading global provider of advanced analytics, technology solutions and contract research services to the life sciences industry. Formed through the merger of IMS Health and Quintiles, IQVIA applies human data science—leveraging the analytic rigor and clarity of data science to the ever-expanding scope of human science—to enable companies to reimagine and develop new approaches to clinical development and commercialization, speed innovation and accelerate improvements in healthcare outcomes. Powered by the IQVIA CORE™, IQVIA delivers unique and actionable insights at the intersection of large-scale analytics, transformative technology and extensive domain expertise, as well as execution capabilities. In addition, global clinical trial data from Citeline, a pharmaceutical clinical trial intelligence company, was leveraged to provide a gold-standard database of curated clinical trial metrics from over 40,000 sources. Publicly accessible data from Clinicaltrials.gov were also analyzed, providing details regarding trial types, locations, and study details.1,8,17
Primary data included both interviews and quantitative survey responses. Sixty- to 90-minute interviews were conducted with a mix of clinical trial personnel (including investigators, site coordinators and raters, n=28), patients (n=8), caregivers (n=8), general practitioners (n=8) and specialists (n=10). In each case, web-assisted telephone depth interviews were conducted in the respondent’s local language. Quantitative survey responses were collected from patients (n=185), caregivers (n=352) and physicians (including both general practitioners and specialists, n=326).9,11,12 The survey was a self-administered, web-based survey customized for each respondent type. Interviewees and survey respondents were recruited naturalistically according to pre-determined screening criteria via a commercial vendor.
Together, these sources helped to inform a quantitative model of the patient journey to an AD clinical trial. This model leveraged literature research, clinical trial data, interview findings and survey responses to estimate the number of patients / subjects flowing through each step in the patient journey and quantify the impact of each barrier identified.
Appendix B: Glossary of Abbreviations
AD – Alzheimer’s disease
CSF – Cerebrospinal fluid
CRO – Contract research organization
CT – Computerized topography scans
DMT – Disease-modifying therapy
GP – General practitioner
HCP – Healthcare provider
MCI – Mild cognitive impairment; note that patients with MCI show early symptoms of mild memory issues/ decline, and many trials often require amyloid-beta positivity (defined as prodromal AD or MCI due to AD)
MRI – Magnetic resonance imaging
PET – Positron emission tomography
PI – Principal investigator
SMT – Symptom-modifying therapy

You must be logged in to post a comment.