Key Takeaways
- Once a COVID-19 vaccine is developed, achieving universal immunization will require a cohesive, multi-faceted vaccination strategy across the U.S. healthcare system.
- Today, vaccination rates for adults under 65 are well below public health targets. Incentivizing younger adults to get recommended vaccines poses a challenge.
- Public health officials and policymakers should prioritize educational interventions for the public and providers, improve access, and reduce cost.
- Finally, community pharmacists can play a vital role in the COVID-19 vaccination effort. To maximize their potential, state practice laws concerning vaccine prescribing authority should be uniform across states and written to automatically include new vaccines once they are approved by the Food and Drug Administration.
Abstract
A vaccine for the novel coronavirus disease 2019 (COVID-19) is a key tool for bringing an end to the outbreak. However, once the vaccine is developed, successful deployment will require that our healthcare system be fully prepared to deliver the vaccine quickly and universally. Several obstacles could impede successful delivery. While childhood vaccination rates for most serious ailments are in the 90th percentile, vaccination rates for adults are well below public health targets. Less than 50 percent of adults aged 19-64 years get a flu shot every year. Adults ages 18–64 with risk factors achieve a pneumococcal vaccination rate of only 24 percent.
Preparations to deliver a COVID-19 vaccine must address factors such as cost and convenience that keep younger adults from being vaccinated. Administering the vaccine at community pharmacies may be the lowest-cost and most convenient alternative. Roughly 9 out of 10 Americans live within five miles of a pharmacy; community pharmacies provide patients with vaccination services without an appointment and offer extended hours of service. However, regulations allowing pharmacist-administered vaccines vary by state, and changes to such regulations will require working through bureaucratic and legal barriers.
There are a number of actions that could be taken now to be ready for quick and universal immunization of the U.S. population for COVID-19. Pharmacists should be integrated into public health planning for vaccine responses to pandemics. For rapid and widespread scalability of a mass immunization campaign, state laws governing pharmacists’ ability to independently immunize patients should be expanded and harmonized to include all Food and Drug Administration-approved adult vaccines. Clinical data systems must link pharmacists, doctors and insurers. In addition, health insurance must fully cover the cost of the vaccination. Now is the time to work on these preparations for COVID-19 and other vaccinations important for public health.
Introduction
Development of a vaccine for the novel coronavirus disease 2019 (COVID-19) is advancing rapidly. To ensure that we can quickly immunize the population once a vaccine is approved, significant challenges must be overcome. This paper describes impediments to universal vaccination that exist today and details policy changes that must be initiated now to facilitate universal COVID-19 vaccination.
The Status of Vaccinations in the United States
Much of the success of vaccines in the U.S. has been achieved through the mandatory vaccination of children, which is often linked to requirements for school enrollment. In 1997, more than 90 percent of U.S. children received three or more doses of the diphtheria, tetanus and pertussis (DTaP) vaccine, the polio vaccine and the Hib vaccine. Over 90 percent of children had at least one dose of the vaccine for measles.[1] This success was despite resistance by a small fraction of parents concerned with the long-term safety of childhood vaccines, which led to periodic, small, localized outbreaks of vaccine-preventable diseases (VPDs) in children.[2]
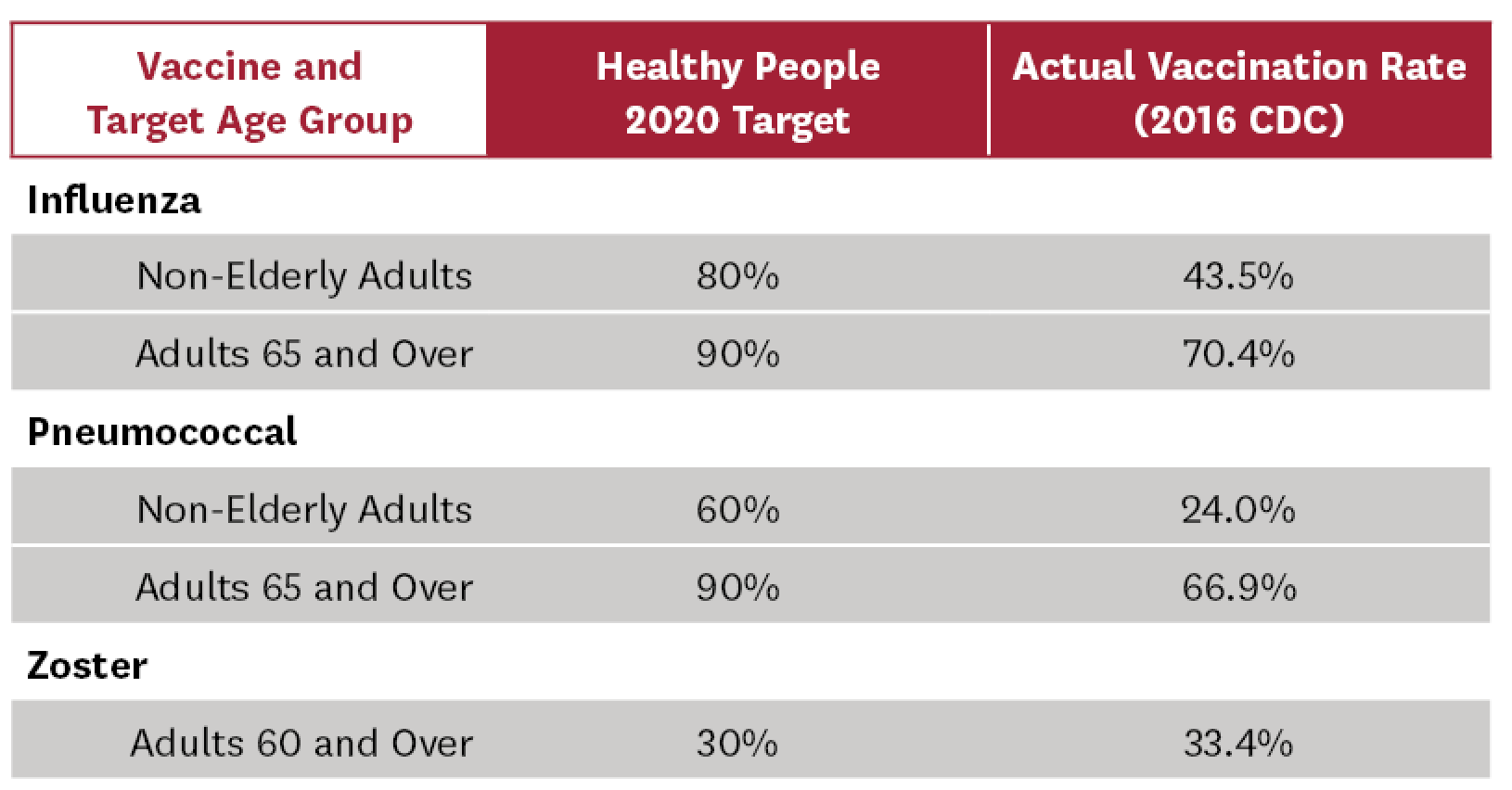
Adult vaccination rates remain well below target rates set by Healthy People 2020[3] and vary significantly across vaccine type and other demographics (age, race/ethnicity).[4] These shortfalls have contributed to more than 40,000 Americans dying from VPDs every year.[5] Table 1 summarizes current vaccination rates compared to Healthy People 2020 targets.
Table 1: Adult Vaccination Rates in the United States[3,6]
Factors That Reduce Adult Vaccination Rates
Planning for the quick and universal application of a future COVID-19 vaccine must account for the factors that have limited adult vaccination rates in the past. An adult’s decision to be vaccinated depends on their perceived risk of being infected, the consequences of an infection, and the direct and indirect costs of being vaccinated.
Low vaccination rates for adults under 65 indicate that healthy, younger adults do not believe they are at high risk for infection and/or the expected cost of the infection is not sufficient to offset the sure cost of vaccination. This calculation changes with age and with the emergence of co-morbidities that increase the cost of being infected. Current flu vaccine recommendations for patients over 65 provide clear evidence of these factors.
In the case of COVID-19, the clinical consequences of an infection for healthy young adults may be relatively mild. As of April 21, 2020, only 0.9 percent of all COVID-19 deaths occurred in patients under 35, while 91.6 percent of deaths occurred in patients over 55.[7] The risk of becoming infected with COVID-19, relative to the annual flu, is not yet known. This implies that, a year from now, when a vaccine may be available and the pandemic may have faded from the headlines, the direct and indirect costs of being vaccinated for COVID-19 must be relatively low to entice participation by young adults.
Conversely, the elderly and individuals with health issues will benefit significantly from herd immunity if vaccination against the virus is universal and quickly achieved. So the suboptimal rates for routine vaccination of adults under 65, as reported above, are of concern. The annual flu results in the deaths of thousands of frail patients annually due in part to vaccination rates failing to meet goals set by the Centers for Disease Control and Prevention (CDC).[8-9] Moreover, achieving universal COVID-19 vaccination will be challenging due to the uneven impact of the virus, coupled with current trends that show routine vaccination rates vary significantly by state,10 urban versus rural areas,11 and race and ethnicity.[12-13]
Preparing U.S. Adults for a COVID-19 Vaccine
What must our healthcare system do to quickly vaccinate the U.S. population when a safe and effective COVID-19 vaccine becomes available?
Mandate vaccinations: Any effort to mandate adult COVID-19 vaccination would be difficult to enforce due to cultural and legal challenges. Adult mandates do not enjoy the natural “choke point” used for mandated childhood vaccinations though the public school system. Childhood vaccines often provide lifetime immunity for serious diseases. Despite these lifelong benefits, mandates for childhood vaccination face significant opposition by a minority of parents who are strongly opposed to vaccinations.[2] Finally, the data are not clear that mandated vaccination with a new COVID-19 vaccine is warranted for all children under 18 or for young adults.[7] The epidemiologic data concerning transmission of the virus from younger patients to high-risk patients once a vaccine is available are not yet available, and mandatory flu vaccinations have never been implemented.
Implement public health education interventions: Changing public perceptions about the importance of vaccinations will be critical if a future COVID-19 vaccine is to be quickly and universally implemented. An important factor influencing vaccine uptake is the provider’s attitude toward vaccination. A 1988 CDC report found that almost 90 percent of patients were vaccinated when both the provider and patient had a positive attitude toward immunization. A provider recommendation resulted in nearly 70 percent of patients with no vaccination plans receiving the vaccine. However, if the patient had positive attitudes toward immunization but the provider did not advocate for vaccination, only 8 percent were immunized.[14]
A program launched in 1987 to educate healthcare providers on the importance of the influenza vaccine increased immunization rates from 10 to 30 percent in two years; introducing standing vaccination orders at the physician’s office increased vaccine uptake to more than 80 percent in the following two years.[15]
The community pharmacist’s role in communicating to patients the importance of being vaccinated is well recognized. In 2007, the Advisory Committee on Immunization Practices endorsed standing-order programs for vaccinations by pharmacists and nurses in long-term care facilities, home health agencies, hospitals, correctional facilities and adult workplaces. These programs were found to be more effective than other institution-based strategies in improving vaccination services.[16] The National Vaccine Advisory Committee has acknowledged that immunization programs in nontraditional settings, such as pharmacies, increase vaccination rates, especially for medically underserved adults. Other strategies for increasing rates include provider recommendations and standing orders that allow other trained healthcare providers to initiate discussion, educate patients and provide the vaccination if indicated.[17]
A 2013 study used 137 pharmacy students to provide a Zostavax vaccine education intervention. The intervention resulted in 343 of 501 unvaccinated patients receiving the Zostavax vaccination during the same pharmacy visit.[18]
Finally, a large national pharmacy chain found that pharmacists were able to identify at-risk patients who required a pneumococcal vaccination. This intervention increased the rate of pneumococcal vaccination for the 1.3 million at-risk patients receiving the pneumococcal vaccine to nearly 5 percent, which exceeded the benchmark vaccination rate of 2.9 percent in a comparator group receiving traditional care.[19]
Improve access and reduce patient cost: As with all consumer products, demand for adult vaccinations depends on the direct and indirect costs of being vaccinated. This is especially important for young adults for whom routine vaccinations provide limited value. Several studies have documented that community pharmacies have significant advantages over other vaccination sites with respect to direct costs. Results from these studies are summarized in Table 2.[20-21]
Table 2: Average Total Cost of Vaccine by Type (Community Pharmacy, Physician’s Office, Other Medical Settings)[20-21]
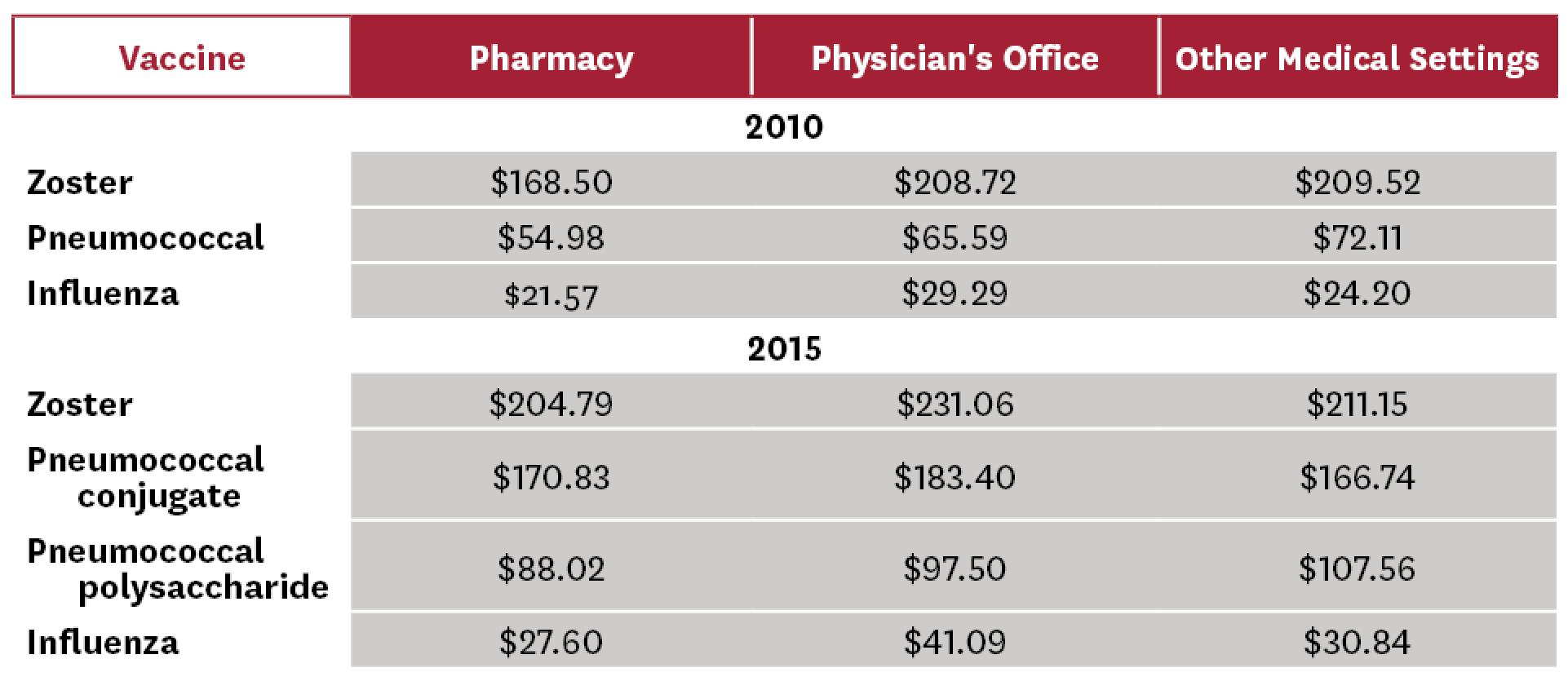
Other studies found differences in indirect costs and consumer preference that favor pharmacies. Burson et al. determined that pharmacies were more effective sites of vaccination than physicians’ offices due to decreased patient waiting time.[22] Shah et al.[23] assessed the patient’s perspective on advantages and disadvantages of being vaccinated at a pharmacy as opposed to a physician’s office for adolescent vaccinations. While most parents agreed that pharmacies provided easier access and more convenient hours, some believed that the doctor’s office provided a better healthcare environment, privacy and safety.[23] Poulose et al.[24] conducted a telephone survey in 2015 and found that 97 percent (n = 233) of patients viewed receiving vaccinations in a pharmacy to be “somewhat pleasant” or “very pleasant.” More importantly, 92 percent of the patients said that they were likely to come back to the pharmacy for an influenza vaccine the following year. Additionally, 69 percent of patients said that they would like to see pharmacists administer other vaccines in the future.[24]
In 2014, Papastergiou et al. studied patient satisfaction from receiving vaccines from a pharmacist.[25] Of 1,502 respondents, 82 percent reported being “very comfortable” with receiving vaccines in a pharmacy and 92 percent reported being “very satisfied” with pharmacists’ administration and injection techniques. In addition, 28 percent of patients said that if it were not for pharmacist-administered vaccines, they most likely would not have gotten vaccinated that year.
In Nova Scotia, Canada, Isenor et al. assessed patients’ vaccine experience using a quality assurance questionnaire.26 Results indicated that nearly 72 percent (n = 6,530) of patients preferred to receive their vaccine from a pharmacy as opposed to the 11 percent who preferred a physician’s office. Patients reported that pharmacy vaccine services were more convenient due to walk-in options, locations and hours. Many patients stated that the pharmacy was less stressful, that they did not feel rushed and that pharmacies offered less contact with other sick people.
Given the above data, it is not surprising that Goad et al.[27] found that a significant proportion of vaccinations provided by a national community pharmacy chain were delivered on nights, weekends and holidays (30.5 percent) when physicians’ offices are closed, and that an additional 17.5 percent of vaccines were delivered by these pharmacies during lunch hours to accommodate the schedules of working adults.
Preparing to Provide the COVID-19 Vaccine in Community Pharmacies
The CDC already recognizes the role of pharmacists in expanding access to vaccines, not only on a routine basis but also in the context of pandemic planning and response. This is reflected in the training that pharmacists now receive to deliver vaccinations. As early as the mid-1990s, the American Pharmacists Association (APhA) began continuing education programs to train pharmacists to administer vaccines. This was followed by a change in accreditation standards for schools of pharmacy in 201628 that mandated vaccination-delivery training for all new pharmacists. As of December 2017, pharmacists are authorized to administer immunizations in all 50 states, and over 320,000 pharmacists have completed the APhA certificate in immunization delivery.[28-32] As a result, community pharmacies played an integral role in the 2009 H1N1 influenza pandemic and studies of that response show that early inclusion of pharmacies could have significantly reduced the time necessary to vaccinate a majority of U.S. adults.[33,34] A recent projection by researchers at Johns Hopkins University estimated that adding community pharmacies to the list of flu vaccine providers would reduce the average number of flu cases per year by 11.9 million, reduce up to 94,307 deaths and save $1 billion in direct healthcare costs.[35] While the U.S. may have enough trained pharmacy personnel to quickly implement a universal COVID-19 vaccination campaign, issues remain that require attention now to speed the response once a vaccine is available.
Adjust State Practice Regulations: State legislation concerning the provision of vaccines by pharmacists without restrictions is uneven. Schmit and Penn[29] found that, as of 2016, 10 states allow pharmacists to independently administer vaccines and five states do not specifically grant prescriptive authority but do not expressly prohibit it either. The remaining 35 states allow pharmacists to assess a patient’s immunization status and administer vaccinations according to a protocol approved by a physician or institution. As of January 2019, our review of the websites listed above found that all 50 states allow pharmacist to immunize some or all vaccines recommended by CDC for adult use. Pharmacists in 18 states are granted prescriptive authority for certain vaccines and ages; the remaining states allow vaccinations only under protocol or with a physician’s prescription. Furthermore, pharmacists in all 50 states and the District of Columbia can administer the influenza, zoster, pneumococcal, meningococcal and Tdap/Td vaccines.[31-32]
Full prescriptive authority for vaccinations by community pharmacists must become uniform across all states if a COVID-19 vaccine is to quickly achieve universal coverage. Even if the goal of uniform vaccination authority is achieved, each state’s pharmacy practice law should be written to automatically include new vaccines once they are approved by the Food and Drug Administration or recommended by the CDC. Any delay in a pharmacist’s ability to administer a newly approved COVID-19 vaccine will only impede target vaccination rates throughout the nation.
Provide Complete Data on Preexisting Conditions and Vaccination History: Accurate, real-time data covering a patient’s clinical and vaccination history is a major impediment to the expansion of pharmacist-provided vaccinations. Complete data will be particularly important if the COVID-19 vaccine is initially limited in supply and rationed to high-risk patients. Physicians’ offices do not provide real-time data to pharmacies unless the physician and pharmacy are part of a larger organization. For example, Kaiser Permanente generally owns its pharmacies and provides outpatient pharmacists with direct links to a patient’s electronic medical record. Achieving this standard of data availability for all community pharmacies would facilitate electronic prescribing and monitoring of drug therapy.
Immunization information systems (IIS) are intended to bridge the data gap between pharmacies and other healthcare providers. The American Immunization Registry Association (AIRA) surveyed numerous pharmacists nationwide in 2014 regarding their familiarity with and use of IIS. The assessment also evaluated challenges that may impact the successful interfacing of pharmacies and IIS. AIRA found that data-quality issues (specifically those associated with demographic data), lack of unique patient identifiers, variations in patients’ names and lack of patient addresses resulted in patient matching and duplication issues.[36]
Conclusion
Vaccines are one of the most cost-effective public health interventions, yet thousands of Americans die each year from VPDs. Today, as we deal with the COVID-19 pandemic and prepare for a potential vaccine, we must quickly implement changes to vaccine policies to ensure the quick and universal provision of a COVID-19 vaccine once it is available.
The preparedness of the U.S. to quickly deliver a COVID-19 vaccine is being discussed in the media.[37] The American Disease Prevention Coalition recently published a statement that comes to many of the same conclusions as this paper.[38] Community pharmacists can play a vital role in the COVID-19 vaccination effort. Roughly 9 out of 10 Americans live within five miles of a pharmacy with the capacity to provide vaccinations during extended business hours and without an appointment. This ease of access will be especially important for younger, healthier adults for whom annual flu immunization rates are extraordinarily low. The evidence reviewed in this paper shows that community pharmacy-based immunizations are the lowest-cost alternative for providing vaccinations.
Authorizing unhindered access to the COVID-19 vaccine in community pharmacies should be a key policy goal as we prepare for its development and approval. A major obstacle is the significant variation in state laws restricting a pharmacist’s ability to immunize patients. Policymakers must act now to harmonize state pharmacy-practice laws. These reforms will help facilitate vaccination against COVID-19 and all the other vaccinations that are so important for public health.
References
1. Centers for Disease Control and Prevention. Achievements in public health, 1900–1999: impact of vaccines universally recommended for children – United States, 1990-1998. MMWR. 1999;48(12):243-48.
2. Phadke VK et al. Association between vaccine refusal and vaccine-preventable diseases in the United States: a review of measles and pertussis. JAMA. 2016 Mar 15;315(11):1149-58.
3. https://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives, IID-12.5 and IID-12.7
4. Centers for Disease Control and Prevention. Estimates of influenza vaccination coverage among adults – United States, 2017–18 flu season. 2018 Nov. https://www.cdc.gov/flu/fluvaxview/coverage-1718estimates.htm.
5. Reid KC, Grizzard TA, Poland GA. Adult immunizations: recommendations for practice. Mayo Clin Proc. 1999;74:377-84.
6. Centers for Disease Control and Prevention. Vaccination coverage among adults in the United States, National Health Interview Survey, 2016. 2018 Feb. https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/pubs-resources/NHIS-2016.html.
7. Centers for Disease Control and Prevention. Provisional Death Counts for Coronavirus Disease (COVID-19). 2020 Apr 22. https://www.cdc.gov/nchs/nvss/vsrr/covid19/index.htm
8. National Vaccine Advisory Committee. A pathway to leadership for adult immunization: recommendations of the National Vaccine Advisory Committee. Public Health Rep. 2012 Jan-Feb;127(Suppl 1):1-42.
9. U.S. Department of Health and Human Services. Healthy People 2020. Washington, D.C. 2011.
10. Centers for Disease Control and Prevention. 2017 Adult Vaccination Coverage General Population Dashboard. 2019 May 2. https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/data-reports/general-population/dashboard/2017.html.
11. Talbert J, Schadler A, Freeman P. Rural/urban disparities in pneumococcal vaccine service delivery among the fee-for-service Medicare population. Rural and Underserved Health Research Center. 2018(4). https://uknowledge.uky.edu/cgi/viewcontent.cgi?article=1003&context=ruhrc_reports.
12. Lu PJ et al. Racial and ethnic disparities in vaccination coverage among adult populations in the U.S. Am J Prev Med. 2015;49(6 Suppl 4):S412-25. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5824643.
13. U.S. Department of Health and Human Services National Vaccine Program Office. National Adult Immunization Plan. 2016. https://www.hhs.gov/sites/default/files/nvpo/national-adult-immunization-plan/naip.pdf
14. Centers for Disease Control and Prevention. Adult immunization: knowledge, attitudes and practices — DeKalb and Fulton Counties, Georgia, 1998. MMWR 1988;37:657-61.
15. Nichol KL. Ten-year durability and success of an organized program to increase influenza and pneumococcal vaccination rates among high-risk adults. Am J Med. 1998;105:385-92.
16. Fiore AE, Shay DK, Haber P, Iskander JK, Uyeki TM, Mootrey G et al.; Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep. 2007;56(RR-6):1-54. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5606a1.htm.
17. Stinchfield PK. Practice-proven interventions to increase vaccination rates and broaden the immunization season (review). Am J Med. 2008;121(7 Suppl 2):S11-21.
18. Teeter BS, Garza KB, Stevenson TL, Williamson MA, Zeek ML, Westrick SC. Factors associated with herpes zoster vaccination status and acceptance of vaccine recommendation in community pharmacies. Vaccine. 2014;32(43):5749-54.
19. Taitel M, Cohen E, Duncan I, Pegus C. Pharmacists as providers: targeting pneumococcal vaccinations to high risk populations. Vaccine. 2011;29(45):8073-76.
20. Singhal PK, Zhang D. Costs of adult vaccination in medical settings and pharmacies: an observational study. Journal of Managed Care Pharmacy. 2014;20(9):930-36.
21. Johnson K, Yang X, Ou W. Pin18. Costs of adult vaccination in medical settings and pharmacies: an observational study. Value in Health. 2019;22(Suppl 2):S197.
22. Burson R, Buttenheim A, et al. Community pharmacies as sites of adult vaccination: a systematic review. Hum Vaccin Immunother. Hum Vaccin Immunother. 2016 Dec;12(12):3146-59.
23. Shah D, Marciniak M et al. Pharmacies versus doctors’ offices for adolescent vaccination. Vaccine. 2018 Jun;36(24):3453-59. https://doi.org/10.1016/j.vaccine.2018.04.088.
24. Polouse S, Cheryian E et al. Pharmacist-administered influenza vaccine in a community pharmacy: a patient experience survey. Can Pharm J. 2015 Mar;148(2):64-67.
25. Papastergiou J et al. Community pharmacist-administered influenza immunization improves patient access to vaccination. Can Pharm J (Ott). 2014 Nov;147(6):359-65.
26. Isenor JE, Wagg AC, Bowles SK. Patient experiences with influenza immunizations administered by pharmacists. Hum Vaccin Immunother. 2018 Mar 4;14(3):706-11.
27. Goad JA, Taitel MS, Fensterheim LE, Cannon AE. Vaccinations administered during off-clinic hours at a national community pharmacy: implications for increasing patient access and convenience. Ann Fam Med. 2013 Sep-Oct;11(5):429-36.
28. Accreditation Council for Pharmacy Education. Accreditation standards and key elements for the professional program in pharmacy leading to the Doctor of Pharmacy degree (“Standards 2016”). Guideline 14.3. 2015. https://www.acpe-accredit.org/pdf/Standards2016FINAL.pdf.
29. Schmit CD, Penn MS. Expanding state laws and a growing role for pharmacists in vaccination services. J Am Pharm Assoc. 2017 Nov-Dec;57(6):661-69.
30. APhA. APhA 2017 annual report. https://www.pharmacist.com/sites/default/files/audience/2017_AnnualReport.pdf.
31. APhA/NASPA. Pharmacist administered vaccines: based upon APhA/NASPA Survey of State IZ Laws/Rules. 2019 Jan. https://media.pharmacist.com/practice/IZ_Authority_012019.pdf.
32. Schmit C, Reddick A. Pharmacist vaccination laws. The Policy Surveillance Program. 2016 Jan. http://lawatlas.org/datasets/pharmacist-vaccination.
33. Fitzgerald TJ, Kang Y et al. Integrating pharmacies into public health program planning for pandemic influenza vaccine response. Vaccine. 2016 Nov;34(46):5643-48.
34. Koonin LM, Beauvais DR Shimabukuro T, Wortley PM, Palmier JB, Stanley TR et al. CDC’s 2009 H1N1 vaccine pharmacy initiative in the United States: implications for future public health and pharmacy collaborations for emergency response. Disaster Med Public Health Prep. 2011 Dec;5(4):253-5.
35. Bartsch SM, Taitel MS, DePasse JV, Cox SN, Smith-Ray RL, Wedlock P et al. Epidemiologic and economic impact of pharmacies as vaccination locations during an influenza epidemic. Vaccine. 2018 Nov;36(46):7054-63.
36. American Immunization Registry Association. Survey of immunization reporting to immunization systems by major U.S. pharmacies: a summary of the methods, successes and challenges of pharmacy-IIS interfaces. 2014 Jan.
37. Weingarden W. Expand pharmacists’ authority to promote access to forthcoming COVID-19 vaccine. Forbes. 2020 Mar 31.
38. American Disease Prevention Coalition. Pharmacists are front line partners in public health: expanded authority is needed to administer vaccine for COVID-19. 2020 Mar 31. https://www.vaccinesshouldntwait.org/wp-content/uploads/2020/03/ADPC-Statement-3_31_20.pdf.
You must be logged in to post a comment.