As the U.S. population ages, costs of care to support persons living with dementia are rapidly mounting. In 2022, medical and unpaid care costs for the estimated 6.5 million people living with dementia—about 1 in 9 Americans aged 65 and older—are expected to total more than $592 billion. By 2050, the number of people living with dementia is projected to nearly double to 12.7 million, with costs rising to $1.5 trillion. For an individual with dementia, total lifetime care costs are estimated at more than twice the costs for an individual without dementia. In addition to bearing these costs, people living with dementia and their care partners often experience declines in quality of life, overall health and physical function, and social and workforce engagement.
But this disease—and its costs—aren’t equally borne by all populations. Studies indicate that, compared to older non-Hispanic white adults, older Black adults are about twice as likely to have dementia, and older Hispanic adults about one and one-half times as likely. Researchers affiliated with the University of Southern California’s (USC) Minority Aging Health Economics Research Center (USC-AD RCMAR) and the Center for Advancing Sociodemographic and Economic Study of Alzheimer’s Disease (CeASES ADRD) have analyzed how race/ethnicity, socioeconomic status (SES) and educational attainment across people’s lifetimes alter dementia risk and years spent with dementia. Their findings indicate that the populations at highest risk of dementia also contend with longer years of life with dementia.
Compound costs: How race, ethnicity, SES and education affect years lost to dementia
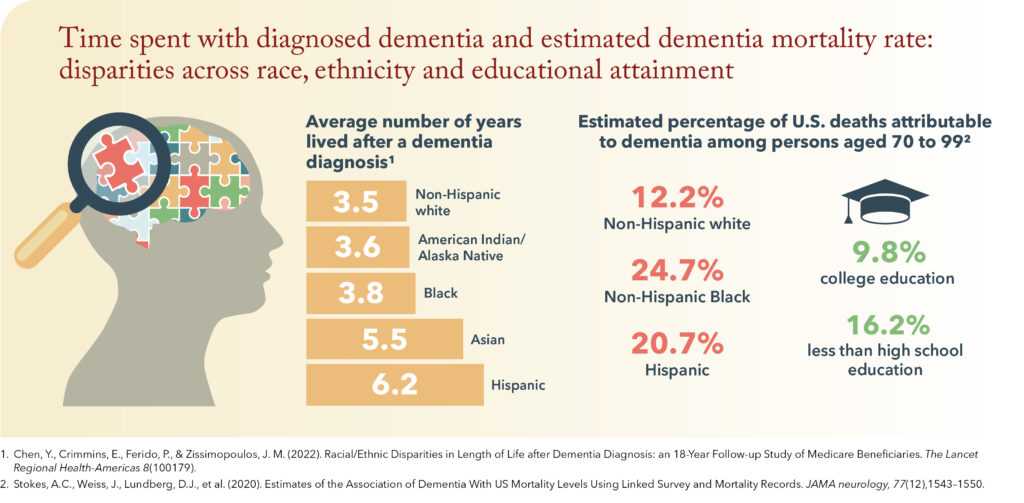
One study conducted by USC-AD RCMAR and CeASES ADRD affiliates found that, on average, Asian, Hispanic and Black individuals were diagnosed with dementia at younger ages than white individuals and lived with dementia longer. For example, Hispanic individuals lived with dementia for an average of 6.2 years whereas white individuals lived with dementia for an average of 3.5 years.
Sign up for Schaeffer Center news
The study’s finding that Black individuals lived longer than white individuals after dementia diagnosis is especially notable given that the overall life expectancy of Black Americans remains lower than that of white Americans—an average difference of 8.3 years for individuals born in 1950 and 3.4 years for those born in 2015.
But race is not the only factor associated with disparities in dementia outcomes. Another study by USC-AD RCMAR and CeASES ADRD-affiliated researchers quantifies how economic and educational disadvantages dynamically affect the risk of severe disease across people’s lifetimes. Using national data from the Health and Retirement Survey (HRS), the researchers found that not only did lower SES at any point increase the number of years spent with dementia, but also disadvantages experienced during three distinct life stages—childhood, early adulthood and later life—added up to create greater risk. For example, male participants with multiple SES disadvantages in childhood, low wealth in later life and less than high school education could be expected to live 6.7 fewer years overall and 8.7 fewer years dementia-free than male participants with no childhood SES disadvantages, high wealth and college education. However, fewer life years attributed to low childhood SES were offset among individuals who despite SES disadvantages achieved high educational attainment as an adult.
The association of worse dementia outcomes with lower SES and educational attainment contributes to racial and ethnic inequities in dementia outcomes. Census data show that the poverty rate in the United States is 11% higher among Black households and nearly 9% higher among Hispanic households than among white households, and that the percentages of individuals with less than high school education are higher in Black and Hispanic populations than in white and Asian populations. These greater economic disparities in childhood SES, educational attainment and SES in later life worsen the impact of dementia on populations that already experience economic disadvantages.
Gaps in reporting mean true disparities may be even greater
A third study indicates that disparities in dementia outcomes may be even larger than national estimates suggest.Using HRS data, researchers found that the estimated number of deaths attributable to dementia was 2.7 times higher than the number of deaths attributed to dementia in cause-of-death records.However, this gap was larger for non-Hispanic Black participants (7.1 times higher) and Hispanic participants (4.1 times higher) than it was for non-Hispanic white participants (2.3 times higher). Educational attainment was also linked to reporting gaps. The gap between estimated and reported dementia deaths was 3.0 times higher for those with less than high school education compared to 2.3 times higher for those who completed high school. That is, socioeconomic and racial and ethnic disparities in deaths attributable to dementia outcomes are larger than current records indicate.
Cascading disparities—and costs
Together, these findings reveal disparities at multiple levels: dementia risk, diagnosis, disease length and deaths attributable to dementia which translate into rapidly mounting economic consequences for those with the fewest resources. Even one additional year of requiring care can incur costs ranging from more than $50,000 for assisted living facilities to more than $100,000 for private nursing home care, in addition to quality-of-life impacts, and lost labor and increased strain on care partners.
The evidence from these studies underscores the need to reduce the unequal impact of dementia. Reducing the amount of time individuals contend with dementia represents an opportunity to not only lower societal costs, but also to address the disproportionate financial and personal repercussions of dementia that fall on economically disadvantaged and underserved populations.
RCMAR (P30AG043073) and CeASES ADRD (P30AG066589). Editorial support was provided by Rose Li and Associates.
References:
- Alzheimer’s Association (2022). 2022 Alzheimer’s Disease Facts and Figures. https://www.alz.org/media/Documents/alzheimers-facts-and-figures.pdf
- CDC (2017). Health, United States, 2017. Table 15. https://www.cdc.gov/nchs/data/hus/2017/015.pdf
- Cha, H., Farina, M. P., & Hayward, M. D. (2021). Socioeconomic status across the life course and dementia-status life expectancy among older Americans. SSM-population health, 15(100921). https://doi.org/10.1016/j.ssmph.2021.100921
- Chen, Y., Crimmins, E., Ferido, P., & Zissimopoulos, J. M. (2022). Racial/Ethnic Disparities in Length of Life after Dementia Diagnosis: an 18-Year Follow-up Study of Medicare Beneficiaries. The Lancet Regional Health-Americas 8(100179). https://doi.org/10.1016/j.lana.2021.100179
- Genworth (2021). Cost of Care Survey. https://www.genworth.com/aging-and-you/finances/cost-of-care.html
- Shrider, E. A., Kollar, M., Chen, F., & Semega, J. (2021). Income and Poverty in the United States: 2020, Current Population Reports, P60-273, U.S. Census Bureau. https://www.census.gov/content/dam/Census/library/publications/2021/demo/p60-273.pdf
- Stokes, A.C., Weiss, J., Lundberg, D.J., et al. (2020). Estimates of the Association of Dementia With US Mortality Levels Using Linked Survey and Mortality Records. JAMA neurology, 77(12),1543–1550. https://doi.org/10.1001/jamaneurol.2020.2831
- United States Census Bureau (2021). Educational Attainment in the United States: 2020. Table 3. https://www2.census.gov/programs-surveys/demo/tables/educational-attainment/2020/cps-detailed-tables/
- Vespa, J., Medina, L., & Armstrong, D. M. (2020). Demographic Turning Points for the United States: Population Projections for 2020 to 2060, Current Population Reports, P25-1144, U.S. Census Bureau. https://www.census.gov/content/dam/Census/library/publications/2020/demo/p25-1144.pdf
- Zissimopoulos, J., Crimmins, E., & St Clair, P. (2014). The Value of Delaying Alzheimer’s Disease Onset. Forum for health economics & policy, 18(1), 25–39. https://doi.org/10.1515/fhep-2014-0013
You must be logged in to post a comment.