Featured Topics
Lead story
Other featured articles
-
In the Wake of the Pandemic: How and Why Housing Plans are Changing in L.A.
Nearly all Angelenos plan to stay put in L.A. Four years ago, they were fleeing in record numbers. What changed in our nation’s most populous county? LABarometer survey data suggest financial constraints kept many low-income residents in place; now, a growing number of these residents appear at risk of displacement.
Posted in -
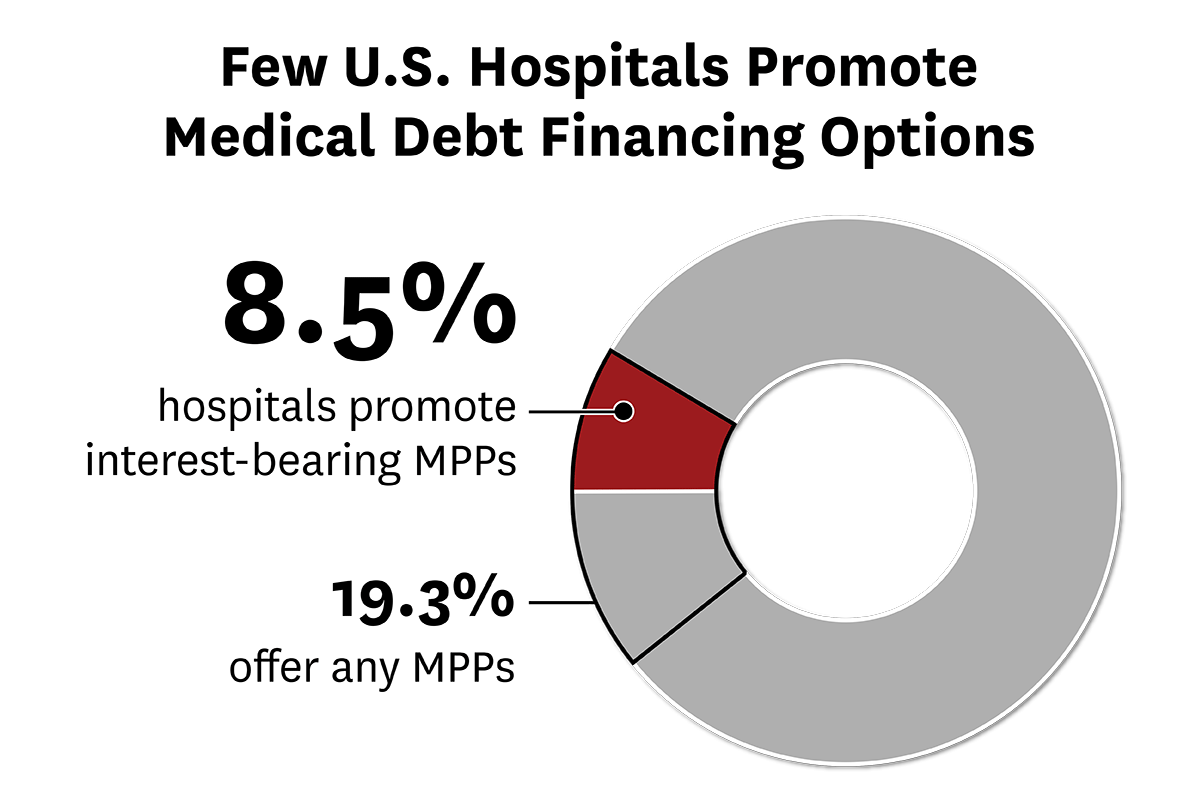
USC Schaeffer Center Study Finds Few Hospitals Promoting Potentially Predatory Medical Payment Products
As Americans struggle to pay off billions of dollars of medical debt, consumer advocates are looking to rein in predatory lending practices. But a recent USC study finds concerns about hospitals offering medical payment products may be overblown.
Posted in -
Buyouts Can Bring Relief From Medical Debt, but They’re Far From a Cure
Local governments are increasingly buying – and forgiving – their residents’ medical debt.
Posted in -
Medicare Part D Plans Increased Restrictions on Drug Coverage
Medicare Part D plans excluded more compounds from coverage or subjected more of them to review before patients can access treatments, USC researchers found.
Posted in
The Leonard D. Schaeffer Center for Health Policy & Economics, one of the nation’s premier policy centers, measurably improves value in health through evidence-based policy solutions, research excellence, and private- and public-sector engagement.
aboutOur Policy Areas
From aging to drug pricing to health disparities, Schaeffer Center experts are advancing research to inform policy. Learn about the areas we work in.
Learn MoreWork & Study at USC
The Schaeffer Center develops innovators for positions in higher education, research, government and healthcare through degree programs and postgraduate opportunities.
Learn More